Topic 6- Periodic Classification – Chemistry Form Two
Periodicity
The Concept of Periodicity
Explain the concept of periodicity
Consider the electronic configuration of the first twenty elements of the periodic table shown in the table below.
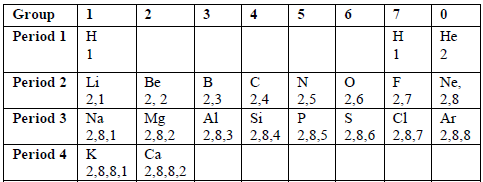
- Positive ions (cations): Across the period; The ionic size does not change, i.e. remains the same, as you move across the period from either direction.
- Negative ions (anions): A negative ion is larger compared to the corresponding neutral atom because on forming an ion, one or more electrons are added to the atom. The added electron(s) is/are repelled by the electron(s) already present in the outermost shell, hence leading to an increase in the size of an atom, even though no new shell is formed. Down the group and along the period: Ionic size increases down the group, and along the period, i.e. from left to right.
- Density-Across the period: Densities decrease across the period from left to right.
- Meting point-Across the period: Melting points of elements decrease across the period from left to right.
- Atomic size-Down the group: Atomic size increases as you move down the group.
- Ionic size- Positive ions (cations)-Down the group: On descending the group, the nuclear charge increases and the number of shells increase by one at each step so, the ionic size also increases. A positive ion is smaller than the corresponding neutral atom because on forming the ion, the metal atom loses both the valency electron(s) and the outermost shell. Valency electron(s) refer(s) to the electron(s) in the outer-most shell of an atom. Any further removal of electron(s) from the ion will decrease the ionic size further.Negative ions (anions)-Down the group and along the period:Ionic size increases down the group, and along the period, i.e. from left to right.
- Density-Down the group: Densities of elements increase down the group.
- Meting point-Down the group: Melting points of elements decrease down the group as the elements become less metallic in nature.
NOTES 2
Constructing the modern periodic table has been a major scientific achievement. The first steps towards working out this table were taken long before anyone had any idea about the structure of atoms. The number of elements discovered increased steadily during the 19th century. Chemists began to find out patterns in their properties.
The Law of Triads
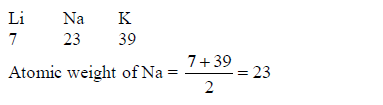
The following are examples of Dobereiner’s triads:(Lithium, Sodium and Potassium)(Calcium, Strontium and Barium)(Chlorine, Bromine and Iodine) and(Iron, Cobalt and Nickel)
The Law of Octaves
In 1863 John Newlands, an English chemist noted that there were many pairs of similar elements. In each pair, the atomic weights differed by a multiple of 8. So, he produced a table with the elements in order of increasing atomic weights, and put forward the Law of Octaves: “If elements are arranged in order of their increasing atomic weights, the properties of the 8th element, starting from a given one, are a kind of repetition of the first element”.
This finding was comparable to the 8th note of music, hence the use of the word “octave”.
This was the first table to show a periodic or repeating pattern of properties. But it was not widely accepted because there were too many inconsistencies. For example, he put copper and sodium in the same group, even though have very different properties. Also iron was placed in the same group as oxygen and sulphur.
The Periodic Law
Dmitri Mendeleev was born in Siberia, Russia, in 1834. By the time he was 32, he was a professor of Chemistry. In 1869 Mendeleev advanced the work done by Newlands and contributed very useful new ideas. He began by listing all the known elements in order of increasing atomic mass. He spotted that elements with similar properties appear at regular intervals or periods down the list. His findings were the basis for the Periodic Law: “The properties of elements are a periodic function of their atomic masses”.
Mendeleev placed similar elements into groups. He realized that not all elements had been discovered. So he left gaps for new ones in the correct places in his table. He also swapped the order of some elements to make them fit better. He predicted the properties of the missing elements from the properties of the elements above and below them in the table. He also listed separately some elements which did not appear to fit into any group i.e. iron, cobalt, nickel, etc.
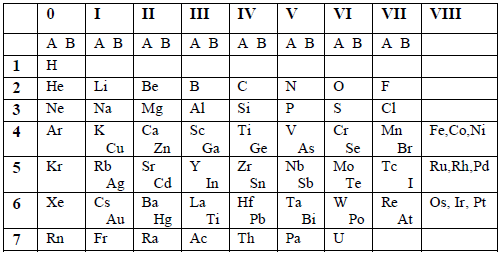
- The table summarized a large amount of information about the elements based on their chemical properties.
- The table was very useful in predicting the existence and properties of undiscovered elements, for which gaps had been left in the table.
- The table was also used in checking relative atomic masses of elements.
- In three cases, pairs of elements had to be included in one group based on inverse order of their atomic weights so as to fit into groups of elements having similar properties. These pairs were argon (39.9) and potassium (39.1), cobalt (58.9) and nickel (58.9); plus tellurium (127.5) and iodine (126.9). This difficulty was resolved when the basis of classification was based on the atomic number instead of the atomic mass.
- The elements that were placed in group VIII formed an incompatible mixture.
- The placing of two different families in one group e.g. K and Cu; Ca and Zn, etc.
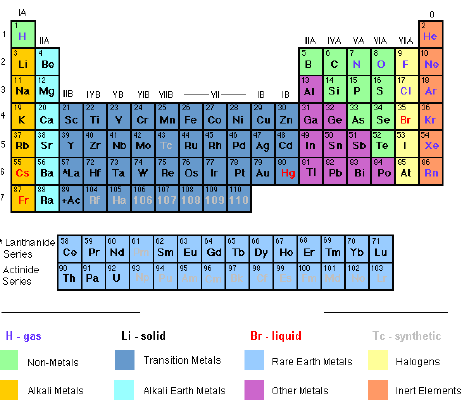
- Group I is often called the alkali metals.
- Group II the alkaline earth metals.
- Group VII the halogens.
- Group 0 the noble gases.
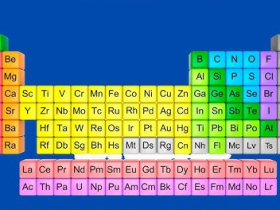
- The elements in the table are placed in order of their atomic numbers instead of their atomic masses.
- There are a total of 18 groups and 7 periods.
- There are 5 blocks of similar elements in the periodic table as shown in figure 6.2.
- The normal (non-transition) elements (groups 1-7) have their outermost shells incomplete, meaning that they can allow additional electrons to enter into their outermost orbital (valency shell). But each of their inner shells is complete.
- The transition metals have their outermost as well as their penultimate (second last) shells incomplete.
- Elements of group 0 (noble gases) have their shells complete. These elements show little reactivity. That is why they wereonce called „inert‟ gases because they are very unreactive; or „rare gases‟ because they were rarely found.
- Gaps left by Mendeleev for undiscovered elements (now occupied by the transition elements and the noble gases) have been filled by the respective elements following their discovery. Man-made elements have also found a place in the periodic table.
- Metals have been clearly separated from non-metals. Metalloids or semi metals (poor metals) have also been included. Metalloids are elements whose properties are intermediate between metals and non-metals. They include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb) and tellurium (Te). In some publications, germanium and antimony are usually classed as poor metals and the rest as non-metals.
































Leave a Reply