TOPIC 4: THERMIONIC EMISSION – PHYSICS NOTES FORM FOUR
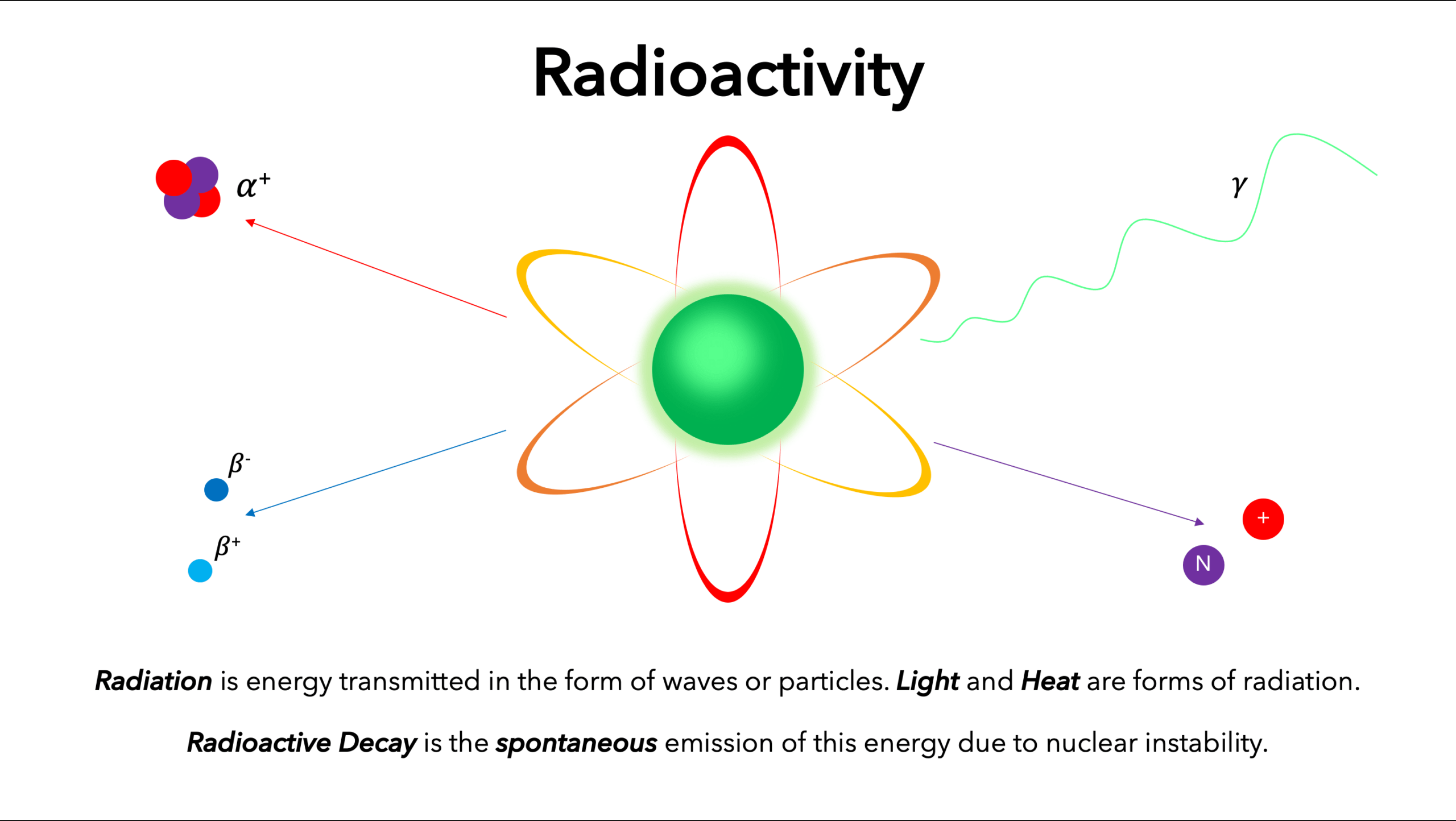
Thermionic emission is the discharge of electrons from heated materials, widely used as a source of electrons in conventional electron tubes (e.g., television picture tubes) in the fields of electronics and communications. The phenomenon was first observed (1883) by Thomas A. Edison as a passage of electricity from a filament to a plate of metal inside an incandescent lamp.
The classical example of thermionic emission is the emission of electrons from a hot cathode into a vacuum (also known as thermal electron emission or the Edison effect) in a vacuum tube. The hot cathode can be a metal filament, a coated metal filament, or a separate structure of metal or carbides or borides of transition metals.
Vacuum emission from metals tends to become significant only for temperatures over 1000 K. The science dealing with this phenomenon has been known as “thermionics,” but this name seems to be gradually falling into disuse.
Cathode Rays
The Production of Cathode Rays

The Properties of Cathode Rays
- Cathode rays travel in straight lines. That is why, cathode rays cast shadow of any solid object placed in their path. The path cathode rays travel is not affected by the position of the anode.
- Cathode rays consist of matter particles, and posses energy by the virtue of its mass and velocity. Cathode rays set a paddle wheel into motion when it is placed in the path of these rays one the bladder of the paddle wheel.
- Cathode rays consist of negatively charged particles. When cathode rays are subjected to an electrical field, these get deflected towards the positively charge plate (Anode).We know that a positively charged body would attract only a negatively charged body, therefore the particles of cathode rays carry negative charge.Cathode rays also get deflected when these are subjected to a strong magnetic field.
- Cathode rays heat the object only which they fall. The cathode ray particles possess kinetic energy. When these particles strike an object, a part of the kinetic energy is transferred to the object. The causes a rise in the temperature of the object.
- Cathode rays cause green fluorescence on glass surface, i.e., the glass surface only which the cathode rays strike show a colored shine.
- Cathode rays can penetrate through thin metallic sheets.
- Cathode rays ionize the gases through which they travel.
- Cathode rays when fall only certain metals such as copper, but rays produced. The X-rays are not deflected by electrical or magnetic fields. X-rays pass through opaque materials such as black paper, but stopped by solid objects such as bones.
- Cathode rays travel with speed nearly equal to that of light.
The Application of Cathode Ray Tube
Televisions
Cathode Ray Oscilloscopes
X-Rays
X-ray tube
Production of X-rays
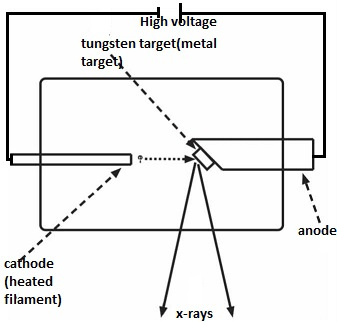
Defference between Soft and hard X-rays and their Production
| Hard x-rays | Soft x-rays |
| They have shorter wavelength(high frequency) | They have longer wavelength |
| They have higher energy | Have less energy |
| Thigher penetrating power | Lower penetrating power |
| Are produced by higher accelerating potential | Produced by lower accelerating potential |
| Have higher velocity | Have lower velocity |
The Properties of X-rays
- They travel in straight lines.
- They readily penetrate matter.
- They are not affected by electric or magnetic fields(they have no charge).
- They cause fluorescence in certain substances.
- They can be detected by photographic emulsion.
- They ionise gases causing the gases to conduct electricity.
The Application of X-Rays in Daily Life
Identify the applications of x-rays in daily life
- In the medical field
- Crystallography
- Astronomy
- X-ray microscopic analysis
- X-ray fluorescence
- Security installations
- Industries


































Leave a Reply