TOPIC 3: RADIOACTIVITY – PHYSICS NOTES FORM FOUR
The Nucleus of an Atom
The Structure of the Nucleus of an Atom
Isotopes
Forces Holding the Nucleus
Stable and unstable atoms
- Binding energy is the net energy that is the result of the balance with the strong force and the repulsive force, and this is the amount of energy that holds the nucleus together.
- A stable atom is an atom that has enough binding energy to hold the nucleus together permanently.
- An unstable atom does not have enough binding energy to hold the nucleus together permanently and is called a radioactive atom.
Natural Radioactivity
The Concept of Radioactivity
The Nuclear Changes due to the Emission of Alpha α, Betaβ and Gammaγ Radiations
Properties of Alpha Rays
- Alpha rays or alpha particles are the positively charged particles.
- Alpha particles have the least penetration power. They cannot penetrate the skin but this does not mean that they are not dangerous.
- Since they have a great ionisation power, so if they get into the body they can cause serious damage. They have the ability of ionising numerous atoms a short distance. It is due to this reason that the radioactive substance that releases alpha particles needs to be handled with rubber gloves. It should not be inhaled, eaten or allowed near open cuts.
Properties of Beta Rays.
- Beta particles are highly energetic electrons which are released from inside of a nucleus.
- They are negatively charged and have a negligible mass.
- Beta particles have a greater penetration power than the alpha particles and can easily travel through the skin.
- Though beta particles have less ionisation power than the alpha particles but still they are dangerous and so their contact with the body must be avoided.
Properties of Gamma Rays
- They have greatest power of penetration.
- They are the least ionizing but most penetrating and it is extremely difficult to stop them from entering the body.
- These rays carry huge amount of energy and can even travel through thin lead and thick concrete.
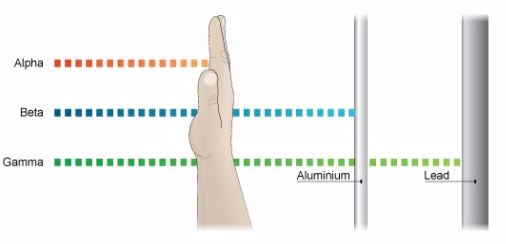
The Detection of α,β andγ Radiations
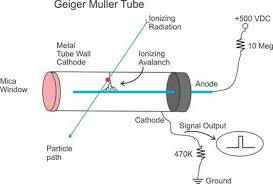
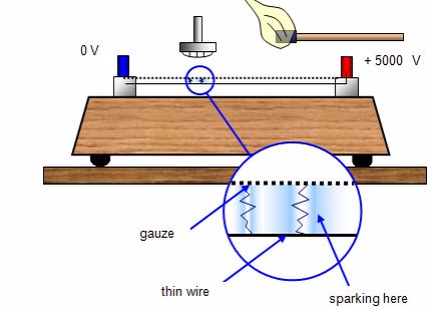
Spark counter
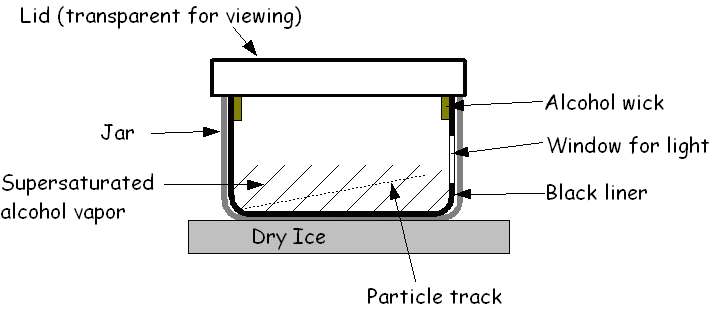
Cloud chamber
- Photographic film
- Bubble chamber
- Gold-leaf electroscope
Half-Life as Applied to a Radioactive Substance
The Half-Life of a Radioactive Element

- N0 is the initial quantity of the substance that will decay (this quantity may be measured in grams, moles, number of atoms, etc).
- N(t) is the quantity that still remains and has not yet decayed after a time t.
- t1⁄2 is the half-life of the decaying quantity.
- τis a positive number called the mean lifetime of the decaying quantity.
- λis a positive number called the decay constant of the decaying quantity.
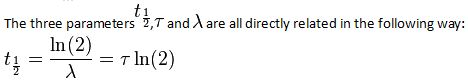

The Application of a Natural Radioactive Substances
Medical Uses
Academic and Scientific Applications
Industrial Uses
Nuclear Power Plants
In agriculture
Artificial Radioactivity
Difference between Natural and Artificial Radioactivity
Methods of Producing Artificial Radioactive Isotopes
- Nuclear activation: Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products and neutrons (in nuclear fission). Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.
- Photonuclear reactions: A photonuclear reaction is a reaction resulting from an interaction between a photon and a nucleus.-During a photonuclear reaction energy of a gamma-ray photon is fully or partially absorbed by the nucleus forcing it into and excited state. From this excited state the nucleus can emit any particle, provided it has enough energy for such a process to occur. Most commonly it will emit a photon, but also a neutron (n), a proton (p) or an alpha (α) particle can be emitted.
Applications of Artificial Radioactivity
Application of artificial radioactivity include:
- Radiation safety:For physicians and radiation safety officers, activation of sodium in the human body to sodium-24, and phosphorus to phosphorus-32, can give a good immediate estimate of acute accidental neutron exposure.
- Neutron detection:One way to demonstrate that nuclear fusion has occurred inside a fusor device is to use a Geiger counter to measure the gamma ray radioactivity that is produced from a sheet of aluminum foil.In the ICF fusion approach, the fusion yield of the experiment (directly proportional to neutron production) is usually determined by measuring the gamma-ray emissions of aluminum or copper neutron activation targets.Aluminum can capture a neutron and generate radioactive sodium-24, which has a half-life of 15 hours[7][8] and a beta decay energy of 5.514 MeV. The activation of a number of test target elements such as sulfur, copper, tantalum and gold have been used to determine the yield of both pure fission and thermonuclear weapons.
- Materials analysis:Neutron activation analysis is one of the most sensitive and accurate methods of trace element analysis. It requires no sample preparation and can therefore be applied to objects that need to be kept intact such as a valuable piece of art. Although the activation induces radioactivity in the object, its level is typically low and its lifetime may be short, so that its effects soon disappear. In this sense, neutron activation is a non-destructive analysis method.
- The potential use of photo-nuclear reactions for a range of applications is described. These are: photo-nuclear transmutation doping of semiconductors, neutron production from electron linacs, quality checking of radioactive waste, fission product incineration, photo-excitation of isomers for dosimetry, and nuclear resonance fluorescence for materials analysis. Initial brief descriptions of atomic and nuclear interactions of photons are given.
Radiation Hazards and Safety
The Effects of Nuclear Radiation on Human Body
These include:
- The size of the dose (amount of energy deposited in the body)
- The ability of the radiation to harm human tissue
- Which organs are affected
- Hair: The losing of hair quickly and in clumps occurs with radiation exposure at 200 rems or higher.
- Brain: Since brain cells do not reproduce, they won’t be damaged directly unless the exposure is 5,000 rems or greater. Like the heart, radiation kills nerve cells and small blood vessels, and can cause seizures and immediate death.
- Thyroid: The certain body parts are more specifically affected by exposure to different types of radiation sources. The thyroid gland is susceptible to radioactive iodine. In sufficient amounts, radioactive iodine can destroy all or part of the thyroid. By taking potassium iodide can reduce the effects of exposure.
- Blood System: When a person is exposed to around 100 rems, the blood’s lymphocyte cell count will be reduced, leaving the victim more susceptible to infection. This is often refered to as mild radiation sickness. Early symptoms of radiation sickness mimic those of flu and may go unnoticed unless a blood count is done. According to data from Hiroshima and Nagaski, show that symptoms may persist for up to 10 years and may also have an increased long-term risk for leukemia and lymphoma. For more information, visit Radiation Effects Research Foundation.
- Heart: Intense exposure to radioactive material at 1,000 to 5,000 rems would do immediate damage to small blood vessels and probably cause heart failure and death directly.
- Gastrointestinal Tract: Radiation damage to the intestinal tract lining will cause nausea, bloody vomiting and diarrhea. This is occurs when the victim’s exposure is 200 rems or more. The radiation will begin to destroy the cells in the body that divide rapidly. These including blood, GI tract, reproductive and hair cells, and harms their DNA and RNA of surviving cells.
- Reproductive Tract: Because reproductive tract cells divide rapidly, these areas of the body can be damaged at rem levels as low as 200. Long-term, some radiation sickness victims will become sterile.
Radiation sickness
Causes
- Accidental exposure to high doses of radiation such as a nuclear power plant accidents.
- Exposure to excessive radiation for medical treatments.
Symptoms
- Bleeding from the nose, mouth, gums, and rectum
- Bloody stool
- Bruising
- Confusion
- Dehydration
- Diarrhea
- Fainting
- Fatigue
- Fever
- Hair loss
- Inflammation of exposed areas (redness, tenderness, swelling, bleeding)
- Mouth ulcers
- Nausea and vomiting
- Open sores on the skin
- Skin burns (redness, blistering)
- Sloughing of skin
- Ulcers in the esophagus, stomach or intestines
- Vomiting blood
- Weakness
First Aid
- Check the person’s breathing and pulse.
- Start CPR, if necessary.
- Remove the person’s clothing and place the items in a sealed container. This stops ongoing contamination.
- Vigorously wash body with soap and water.
- Dry the body and wrap with soft, clean blanket.
- Call for emergency medical help or take the person to nearest emergency medical facility if you can do so safely.
How to Protect yourself from Nuclear Radiation Hazards
Precautions
- Time: An average the procedure time for a diagnostic coronary angiogram is approximately 30 minutes and an interventional procedure PCI or EPS/pacing would take between 90 to 120 minutes. However the fluoroscopic and the cine screening time are highly variable depending on the nature of the procedure and the experience of the operator. The lower the amount of time spent in a radiation area, the lower the exposure will be. Significant reductions can be achieved when an activity is delayed until after cine imaging is completed. Every effort should be made by the operating cardiologist in the cath lab to minimise fluoroscopy and cine screening time.
- Distance:Increasing the distance from the radiation beam decreases the risk of exposure. doubling the distance between the primary beam and operator, reduces the exposure by a factor of four. In addition, the radiation exposure varies according to the angle at which the camera is projected Oblique views (left and right anterior oblique) and steep angulations increase radiation exposure but are often employed to improve visualisation. 60-degree angulations give up to three times the operator dose than 30-degree angulations (11). The second operator or assistant is generally less exposed to radiation compared to the first operator but certainly more at risk than the other staff in the room.
- Shielding: Lead shields and shielding will significantly reduce the risk of exposure but only if appropriately used and in proper working order. Protective equipment includes lead aprons, thyroid collars and leaded glasses. With the newly designed frames and ultra light lenses, protective leaded eyewear is now used by more of the cardiologists and staff in cardiac cath lab. Some cath labs also use overhanging lead screens to prevent radiation exposure to brain. The staff should wear a protective apron of at least 0.25 mm lead equivalent. Protective gloves should be of at least 0.35 mm lead equivalent. All such protective clothing should bear an identifying mark and should be examined at yearly intervals. Defective items should be withdrawn from use.
- Adhering to guideline and protocols: Every unit or work place that deals with ionising radiation should have their own local guidelines and rules for radiation safety. These must be read, understood and strictly adhered to in daily practice. Staff must comply with these local rules in order to insure that the Trust and all their employees do not contravene statutory requirements of the ionising radiation regulations and other relevant legislation.
- Minimising risk of exposure to staff and patients: The occupational limit of radiation exposure in the UK currently is estimated at 20 mSv per year averaged over five consecutive years (5). Every operator who undertakes a cardiovascular procedure in the cath lab is responsible for the amount of radiation exposure to the patient, his or her co-staff and to themselves. In the event of an incident where the patient might have been exposed to inadvertent excess radiation either due to clinical circumstances, malfunctioning of the equipment or operation errors, the radiation protection adviser should be informed of the incident. It is their duty to estimate the radiation dose received by the patient and also advise whether the incident is to be reported.Only essential staff shall be in the cath lab during radiation exposure. All persons not required in the room should leave the room during serial radiographic exposure. The operator shall stand behind a barrier if possible. People who must move around the room during the procedure should wear a wraparound protective garment. When possible, the cardiologist and all other personnel required in the room should step back from the table and behind portable shields during cine and serial radiography procedures. This action can decrease the exposure of the cardiologist and the other nearby personnel by a factor of three or more (10).
Nuclear Fission and Fusion
The Nuclear Fission and Fusion
Nuclear fission
Nuclear fusion
Application of Nuclear Fission and Fusion
- Nuclear power plants to generate electricity for domestic and industrial use.
- In making nuclear bombs.
- In fussion power plants to make electricity.
- To make nuclear weapons such as the hydrogen bombs.


































Leave a Reply