Topic 4- Fuels and Energy – Chemistry Form Two
A fuel is a substance that can be combusted or burnt to release energy as a byproduct. The energy can be in the form of heat, light, electricity, sound etc. This energy can be harnessed to power machines or used for other purposes such as heating or lighting. Combustion is the burning of fuel with energy released as a byproduct. Fuel is a very important substance for the existence of a modern man. Examples of fuels include petroleum products (petrol, diesel, fuel oil, kerosene, spirits, etc), natural gas, coal, wood, charcoal, producer gas, water gas, etc.
Fuel Sources
Identify different sources of fuels
- Wood: wood is obtained from logs or poles of trees. The wood used as fuel in Tanzania is obtained from natural and artificial forests. Wood fuel is mainly used in rural areas where there are no alternative fuels. Wood is also a major source of fuel used by government institutions such as schools, colleges, hospitals, and military institutions.
- Charcoal: This fuel is made by heating certain substances such as wood and bones in a limited supply of air. Wood charcoal is the main source of fuel in urban areas and in some townships.
- Coal: coal used in Tanzania is mined at Kiwira coal mines. It is used indirectly for generating electricity or directly for powering machines in processing and manufacturing industries and factories. The electricity generated from coal is used in such industries as Tanga cement and several other industries in Dar es Salaam.
- Natural gas: This gaseous fuel is mined at Songosongo in Kilwa (Lindi region), located in southern Tanzania. The gas is used as a fuel at homes and in small industries. It is also used to generate electricity that is used in various manufacturing and processing industries. The electricity generated from this gas is also sold to Tanzania Electricity Supply Company (TANESCO) who distributes the energy to its various clients.
- Petroleum products (kerosene, diesel, petrol, fuel oil, fuel gas, etc.) These petroleum fractions are obtained from crude oil by the process of fractional distillation of crude oil (petroleum). Diesel, petrol and oil are used in vehicles and other machines. Kerosene is used in kerosene lamps and stoves for heating at homes and for other general purposes.
Methods of making charcoal
- Cut wood into small pieces.
- Arrange the wood pieces into a pile of wood on the ground.
- Cover the pieces of wood with soil, leaving one open space for setting fire.
- Set fire to the wood and then cover the open space with soil. Make sure that the wood is burning.
- After the wood is burned, uncover the soil and pull out the black solid substance underneath. This is the charcoal.
Coal is formed from the remains of lush vegetation that once grew in warm shallow coastal swamps. The following are the stages in the process of coal formation:
- The dead vegetation collects in the bottom of the swamp. It may start to decay. But decay soon stops, because the microbes that cause it need oxygen, and the oxygen dissolved in the stagnant, warm water is quickly depleted.
- The vegetation is buried under debris.
- Over hundreds of thousands of years, the environment changes. Seas flood the swamps. Heavy layers of sediment pile up on the dead vegetation, squeezing out gas and water and turning it intopeat.
- As the peat is buried deeper, the increasing heat and pressure compress it progressively to form different types of coal.
- As the process continues, the coal gets harder and more compact. Its carbon content also increases, giving different types of coal. Table bellow shows a summary of the stages in the process:
| Name of coal | Carbon content | ||
| Peat | 60% | ||
| Pressure and Heat | Lignite | 70% | Hardness |
| Bituminous coal | 80% | ||
| Anthracite | 95% |
As carbon content increases so does energy given out per unit weight. But hard coal tends to have higher sulphur content, hence likely to cause environmental pollution. When burnt, the sulphur in the coal produces sulphur dioxide gas that is released into the atmosphere, causing air pollution. S(s)+O2(g)->S02(g)
Categories of Fuels
Fuels can be classified into three groups according to the physical state of the fuel. A fuel can be in any of the three states of matter namely, solid, liquid or gaseous state.
Classify fuels according to their states
Solid fuels
- Fuel naturally found in nature: -natural gas -methane from coal mine
- Fuel gas from solid fuels or materials: -gas derived from coal (water gas and producer gas) -gas derived from wastes and biomass (biogas)
- Fuel gas made from petroleum.
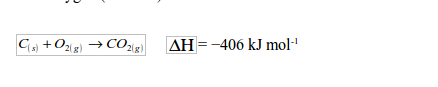

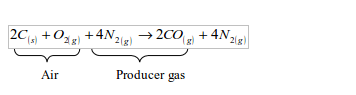


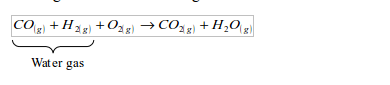
- It should be environmentally friendly (not harm the environment) in the course of its production and use, that is, it should not produce harmful or toxic products such as much smoke, carbon dioxide, carbon monoxide, sulphur dioxides, etc, which pollutes the air.
- It must be affordable to most people i.e. it must be cheap.
- It should not emit or produce dangerous by-products such as poisonous fumes, vapour or gases.
- It should have high calorific value i.e. it must burn easily and produce a tremendous quantity of heat energy per unit mass of the fuel.
- It should be easy and safe to transport, store, handle and use.
- It should be readily available in large quantities and easily accessible.
- It should have high pyrometric burning effect (highest temperature that can be reached by a burning fuel). Normally gaseous fuels have the highest pyrometric effect as compared to liquid and solid fuels.
- It should have a moderate velocity of combustion (the rate at which it burns) to ensure a steady and continuous supply of heat.
- A good fuel should have an average ignition point (temperature to which the fuel must be heated before it starts burning). A low ignition point is not good because it makes the fuel catch fire easily, which is hazardous, while high ignition point makes it difficult to start a fire with the fuel.
- A good fuel should have a low content of non-combustible material, which is left as ash or soot when the fuel burns. A high content of no-combustible material tends to lower the heat value of the fuel.
| Solid and liquid fuels | Calorific value (MJ/kg) |
| Alcohols | |
| Ethanol | 30 |
| Methanol | 23 |
| Coal and coal products | |
| Anthracite (4% water) | 36 |
| Coal tar fuels | 36 – 41 |
| General purpose coal (5-10% water) | 32 – 42 |
| High volatile coking coals (4% water) | 35 |
| Low temperature coke (15% water) | 26 |
| Medium-volatile coking coal (1% water) | 37 |
| Steam coal (1% water) | 36 |
| Peat | |
| Peat (20% water) | 16 |
| Petroleum and petroleum products | |
| Diesel fuel | 46 |
| Gas oil | 46 |
| Heavy fuel oil | 43 |
| Kerosene | 47 |
| Light distillate | 48 |
| Light fuel oil | 44 |
| Medium fuel oil | 43 |
| Petrol | 44.80 – 46.9 |
| Wood | |
| Wood (15% water) | 16 |
| Gaseous fuels at 15ºC, 101.325 kPa, dry | Calorific value (MJ/m3) |
| Coal gas coke oven (debenzolized) | 20 |
| Coal gas low temperature | 34 |
| Commercial butane | 118 |
| Commercial propane | 94 |
| North sea gas, natural | 39 |
| Producer gas coal | 6 |
| Producer gas coke | 5 |
| Water gas carburetted | 19 |
| Water gas blue | 11 |
Measuring the heat given out by fuels
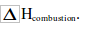
- Pour a measured volume of water into the tin. Since you know its volume you also know its mass (1 cm3 of water has a mass of 1g).
- Weigh the fuel and its container.
- Measure the temperature of the water.
- Light the fuel and let it burn for a few minutes.
- Measure the water temperature again, to find the increase.
- Reweigh the fuel and container to find how much fuel was burned.
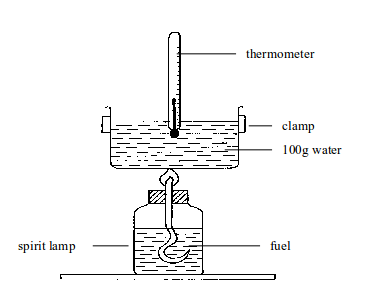
| Ethanol (burned in a spirit lamp) | Butane (burned in a butane cigarette lighter) |
| Results | Results |
| Mass of ethanol used: 0.9g | Mass of butane: 0.32g |
| Mass of water used: 200g | Mass of water used: 200g |
| Temperature rise: 20ºC | Temperature rise: 12ºC |
| Calculations | Calculations |
| Heat given out = or 16.8KJ | Heat given out = = 10080J or10.08KJ |
| The formula mass of ethanol is 46. 0.9g gives out 16.8KJ of energy. So, 46g gives out of energy | The formula mass of butane is 58. 0.32 gives out 10.08KJ of energy. So, 58g gives out KJ of energy |
| So, H combustion for ethanol is -859KJ/mol | So, H combustion for butane is –1827 KJ/mol |
- Pour a known volume of water into a beaker.
- Measure the temperature of the water.
- Fill the spirit lamp with enough ethanol.
- Weight the mass of both the ethanol and the lamp.
- Light the lamp and let it continue burning for a few minutes before putting it off.
- Measure the water temperature again, to find the increase.
- Reweigh the ethanol and its container to find how much ethanol was burned.
- Mass of spirit lamp + ethanol (initially)
- Mass of spirit lamp + ethanol (finally)
- Mass of ethanol burned
- Final temperature of water
- Initial temperature of water
- Rise in temperature of water
- Mass of water

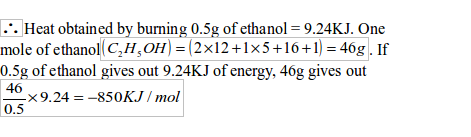
| Fuel | Heat of combustion in KJ/mol | |
| From the experiment | From a data book | |
| Ethanol | -859 | -1367 |
| Butane | -1827 | -2877 |
- Heat loss: Not all the heat from the burning fuel is transferred to the water. Some is lost to the air, and some to the container that holds the fuel.
- Incomplete combustion: In case of a complete combustion, all the carbon in a fuel is converted to carbon dioxide. But here combustion is incomplete. Some carbon is deposited as soot on the bottom of the lamp and some converted to carbon monoxide. For example, when butane burns, a mixture of all these reactions may take place:
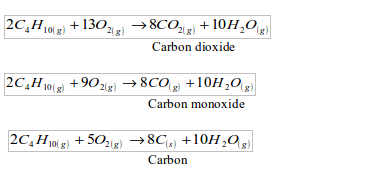
Renewable Energy Biogas
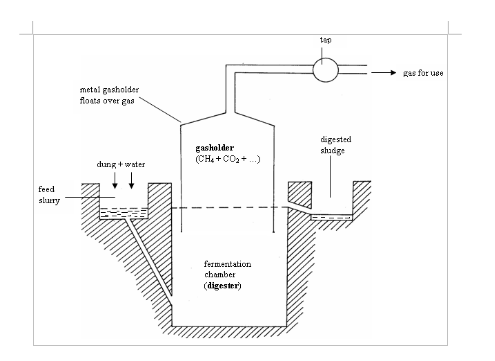
- Biogas does not produce much smoke or ash, which could otherwise pollute the atmosphere or land. When the gas is burned it produces very little smoke and no ash as compared to other sources of fuel such as wood.
- The use of biogas for cooking and heating prevents the cutting down of trees to harvest firewood, or burn charcoal for fuel, a practice that could result to soil erosion, drought, etc. Hence, using the biogas as fuel helps to conserve the environment as no more cutting of trees may be done.
- Using cow dung, poultry manure and other excreta for biogas production helps keep the environment clean because these materials are put into alternative use instead of just being dumped on land, a fact that could lead to pollution of the environment.
- Some biomass employed in biogas production is toxic and harmful. By letting these materials be digested by bacteria, they may be turned into non-toxic materials that are harmless to humans, plants, animals and soil.
- The excreta used for production of biogas produce foul smell if not properly disposed of. Using this excrete to generate biogas means no more bad smell in air.
- Health hazards are associated with the use of sludge from untreated human excreta as fertilizer. In general, a digestion time of 14 days at 35ºC is effective in killing the enteric bacterial pathogens and the enteric group of viruses. In this context, therefore, biogas production would provide a public health benefit beyond that of any other treatment in managing the rural health and environment of developing countries.































Leave a Reply