TOPIC 7: MEASUREMENT OF THERMAL ENERGY – PHYSICS NOTES FORM THREE
Heat Capacity
The Factors which Determine Heat Quality of a Substance
- mass of the substance
- temperature change
- specific heat capacity of the substance
The Heat Capacity
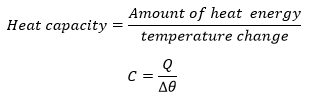
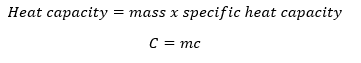
Example 1
Data Given
- The quantity of heat required to change the temperature of a body with mass, mkg byθ degree Celsius is mcθ joules.
- In order to raise the temperature of a body, heat must be supplied to it.
- In order to lower its temperature, heat must be removed from it.
The Specific Heat Capacity
- The mass of the body, M
- The temperature different, ΔT
- The thermal properties of the body.
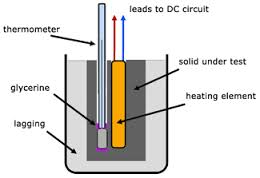
Transfer of Heat
- Calorimeter – Is the special instrument or vessel used for measurement of Heat.
- Calorimeter is highly polished metal can usually made of copper or aluminium.
- It is flitted with an insulating cover in which there are two holes.
- Two holes allow a thermometer and a stirrer to be inserted.
- The stirrer is made of the same metal as that of the calorimeter.
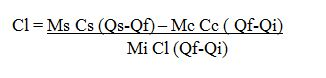
Example 3
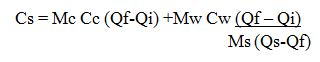
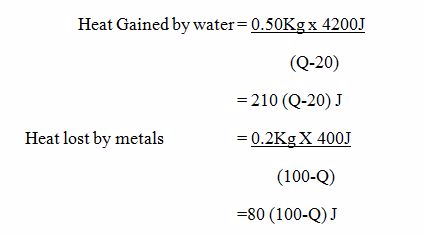
Change of State
The Behaviour of Particles of Matter by Applying Kinetic Theory
Solids, liquids and gases
| In solids the particles | In liquids the particles | In gases the particles |
|
|
|
- the properties of matter
- what happens during physical changes such as melting, boiling and evaporating
The properties of matter
| Solids | Liquids | Gases |
|---|---|---|
|
|
|
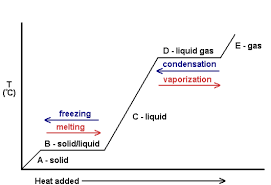
The graph of temperature versus temperature for a Heated.
The Melting Point of a Substance from its Cooling Curve
Deposition: Is the change of the state matter from gas into solid. e.g. Ammonium chloride vapour and Iodine vapour into solid (NH4CI) and (Iodine).
Melting point (m.p) table
Substance |
Melting point (ºC) |
| Copper | 1083 |
| Glass | 1000 – 1400 |
| Iron | 1450 |
| Lead | 327 |
| Pitch | 40 – 80 |
| Mercury | – 39 |
| Platinum | 1775 |
| Tin | 232 |
| Tungsten | 3377 |
The Effect of Impurities on the Freezing Point and the Boiling Point of a Substance
Effect of impurities on Boiling Point
Effect of impurities on freezing point
Conclusion
- The impurities present in a liquid pull its two fixed points away from each other i.e. the freezing point is lowered while the boiling point is raised.
- The depression in freezing point and the elevation in boiling point increases with increase in the concentration of the solute or impurity i.e. these are the colligative properties that depends only on the no. of moles of the solute. They are independent of the nature of the solute.
The Effect of Pressure on the Boiling Point and Freezing Point of a Substance
The Phenomenon of Regelation
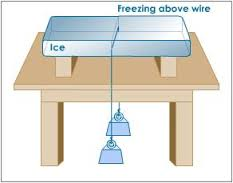
The Concept of Boiling and Evaporation in Respect to the Kinetic Theory of Matter
Evaporating
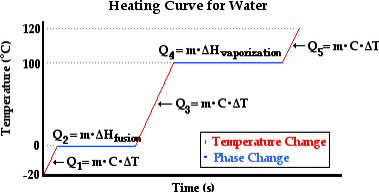
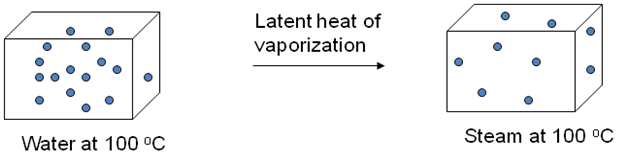
Latent Heat of Fusion and Vaporisation
Latent heat of Vaporization is the heat required to change a liquid into a gaseous state at constant temperature.
- Mass of Beaker = M1 kg
- Mass of Beaker + Water = M2 kg
- Time taken to Boil =t1 Minutes
- Time taken to Boil away = t2 Minutes
- Specific latent heat of = L J / kg Vapor
- Heat gained by steam = (M2 – M1)L
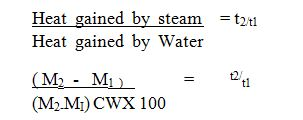
- Mass of Calorimeter + stirrer = M1
- Mass of calorimeter +Water =M2
- Mass of Calorimeter +Water = M3
- Initial Temperature of Water = Q1
- Final temperature of Water =Qf
- Mass of Water = ( M2 – M1 )
- Mass of Ice = ( M3 – M2 )
- CW is the specific heat capacity of water.
- Heat lost by the original water in the calorimeter = (M2 – M1) ( Q1 – QF ) Cw.
- heat lost by calorimeter and stirrer = M1 CC ( Qi – Qf ).
- Cc is the specific heat capacity of the material of the calorimeter.
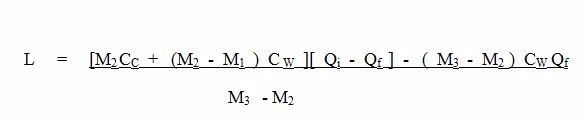
Specific latent Heat of fusion is the amount of heat required to change a unit Mass of solid substance into liquid at constant temperature
| SUBSTANCE | SPECIFIC LATENT HEATOF FUSSION J/ kg |
| Ice | 334400 |
| Naphthalene | 146300 |
| Lead | 24662 |
| Copper | 179740 |
| Aluminum | 317680 |
| Gold | 66880 |
- Specific Heat capacity of ice = 2.1 x 103 J/ KgºC
- Specific Heat capacity of copper = 420 J/ Kg ºC
- Specific Heat Capacity of Water = 4200 j/ Kg º C
How it Works
- The latent heat of Vaporization comes from the air surrounding the coil i.e. from the inside of the freezing g cabinet
- An eclectically driven pump P remove the vapor from A and force it into the heat exchanger C, which is made of copper coils.
- The coils of the heat exchanger are filled with cool fins F
- In the heat exchanger, vapor is compressed by the pump and condensed back to liquid.
- The conversion of vapour into liquid in (c) gives out the latent heat of vaporization, which is conducted away by the fins.
- The condensed liquid is then returned to the evaporator coil (A) through avalve (V) (in this way a continuous circulation of vapour and liquid is set up).
- The rate of evaporation and the degree of cooling is controlled by a thermostat, which switches the pumps motor on and off at intervals.
- The thermostat can be adjusted to give the desired low temperature inside the freezing cabinet where food is preserved.
































Leave a Reply