Topic 3- Soil Chemistry – Chemistry Form Four
Soil Formation
Soil Formation
Soil is formed by the process of weathering. All types of weathering (physical, chemical or biological) result to disintegration of rocks into smaller particles. Air and water enter the space between these particles and chemical changes take place, which lead to the production of chemical substances. Bacteria and plant life soon appear. When plants and animals die, they decay and produce humus. Bacteria and other decomposers play a vital role in the decomposition of plant and animal substrata. The end product of these mechanical, chemical and biological processes is soil.
Therefore, soil can be defined as unconsolidated mineral (inorganic) and organic material on the immediate surface of the earth‟s crust that serves as the medium for plant growth.

The Factors Influencing Soil Formation
- ingesting organic mineral materials e.g. earthworms and millipedes;
- transportation of materials e.g. earthworms, millipedes, termites, beetles, etc; and
- improvement of soil structure and aeration.
- Cultivation of soils for production of food and tree crops, which in many cases has negative effects causing impoverishment of the soil and erosion.
- Indiscrimate grazing, casual burning, cutting of trees, manure and fertilizer use, all of which alter the soil characteristics.
Soil Reaction

- Leaching: Heavy rains may leach bases like Ca2+, Mg2+, K+ andNa+ from the soil to the ground water table, leaving a surplus ofH+(aq) in the soil.
- Soil Microorganisms and root respiration produce carbon dioxide which forms weak carbonic acid with the soil solution.
- Near industrial regions, acid rain (often pH 2–4) may bring sulphuric (IV) acid and nitric (V) acid to the soil.
- Acid mineral fertilizers, like ammonium sulphate (VI) andammonium chloride make the soil solution more acidic due to oxidation and hydrolysis:
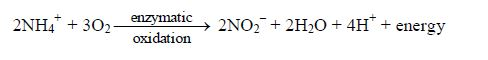
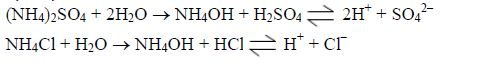
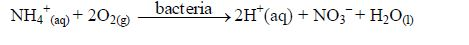

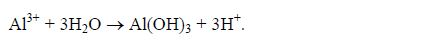
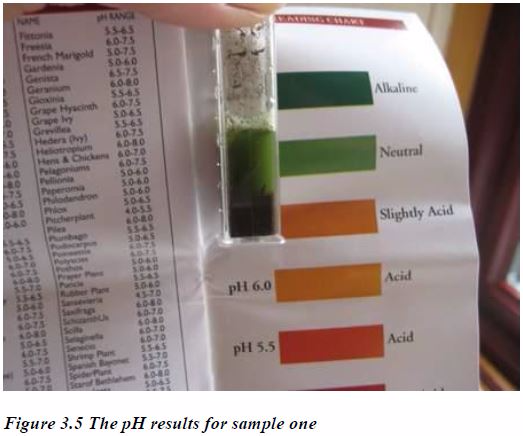
The pH of a Given Soil Sample
The pH of a soil can be tested by using Soil pH Kit. The kitcomprises of equipment and dyes (pigments) that are employed insoil pH determination.

Soil pH can vary in different parts of your garden either naturally or through different types and levels of cultivation. You may be able to see clear differences in colour, texture and humus content. Therefore, aim to take a number of samples from different areas and test each one separately.
2. Collecting the soil samples



| Crop | Preferred pH |
| Irish potato | 4.5 – 6.0 |
| Chicory, parsley | 5.0 – 6.5 |
| Carrot, sweet potato | 5.5 – 6.5 |
| Cauliflower, garlic, tomato | 5.5 – 7.5 |
| Broad bean, onion, cabbage and many others | 6.0 – 7.5 |
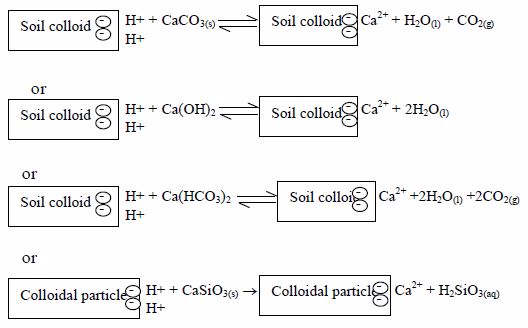
Managing the Soil pH by Using Different Liming Materials
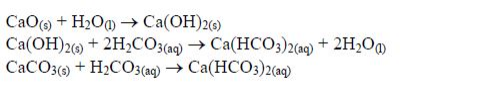
Plant Nutrients in The Soil
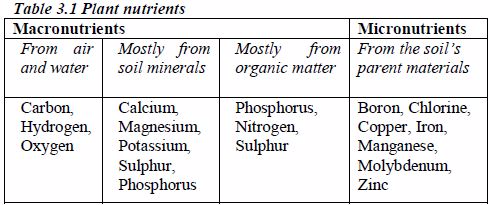
Categorize the essential plant nutrients
MACRO AND MICRONUTRIENTS
Micronutrients, also referred to as trace elements, are those mineral nutrients that are required by plants in smaller amounts. They constitute about 1% of plants‟ requirements.
INTAKE OF NUTRIENTS BY PLANTS
The Functions of Each of the Primary Macronutrients in Plant Growth
AVAILABILITY, FUNCTION AND DEFICIENCY OF PLANT NUTRIENTS
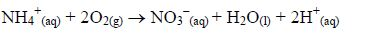
- It is used to build amino acids, nucleic acids, many enzymes, chlorophyll, generally speaking: all proteins.
- It promotes the vegetative growth in plants. It is therefore important in the growth of plants in which leaves are harvested, such tobacco and vegetables.
- It is an essential element in cell division. It is therefore needed for plant growth.
- It increases grain size and protein content in cereals.
- It promotes root growth.
- Phosphorus is an essential component of the genetic material of the cell nucleus (RNA, DNA); also in ADP and ATP, which play a vital role in photosynthesis, amino acid and fat metabolism, etc.
- It increases the grain yield e.g. of millet, sorghum and rice because it promotes the formation of tillers.
- It promotes root growth.
- It strengthens the resistance of plants to diseases.
- Also rhizobia bacteria need it in order to fix nitrogen from the air.
- It hastens plant maturity.
- Dark green colouration
- Purple spots or streaks.
- Stunting, delayed maturity.
- Potassium is an activator of a number of enzymes involved amino acid synthesis and several enzymes concerned with carbohydrate and nucleic acid metabolism.
- Potassium aids in the uptake of other nutrients and in their movements within the plant e.g. potassium ions and nitrate (V) ions may move together.
- Potassium is also important in the metabolism of carbohydrates and translocation of food. Thus, it promotes starch and sugar formation.
- It regulates osmosis in cells, improves tissue formation and assists in protein synthesis.
- It strengthens plant stalk, hence preventing lodging and microbial attack.
- Stunting: First the edges of the older leaves and then areas between veins turn yellow and finally brown. Small, brown necrotic spots develop while the veins are still green.
- Leaf curling and premature leaf fall.
- Calcium is a constituent of cell walls and hence makes the straw stiff and resistant to lodging.
- It is essential for cell division.
- It promotes early root and seed development.
- It regulates the intake of potassium by plants.
- It neutralizes harmful organic acids like ethanedioic (oxalic) acid in plants, thus detoxifying them:
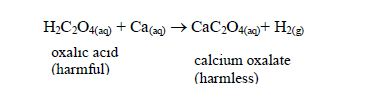
- Magnesium is vital to the production of chlorophyll, because every molecule of chlorophyll contains a magnesium ion at the core of its complex structure. Most of the magnesium in plants is found in either chlorophyll or seeds. A lesser part is distributed in other parts.
- Aids in the translocation of carbohydrates.
- Regulates the uptake of other nutrients.
- Part of the distributed magnesium functions in the enzyme system involved in carbohydrate metabolism.
- Sulphur is a vital part of plant proteins since cystine and methionine are sulphur-containing amino acids.
- Sulphur is also essential for the action of enzymes involved in nitrate (V) production.
THE OTHER MICRONUTRIENTS
- Manganese is a catalyst in the formation of chlorophyll and in many redox reactions, e.g. metabolism of nitrogen, iron, copper, zinc and in vitamin C synthesis.
- Boron aids protein synthesis, regulates the K:Ca ratio in plant tissues and is required for the formation of roots and fruits.
- Copper is involved in respiration and in the nitrogen and iron metabolism.
- Molybdenum is essential in the protein synthesis and for the nitrogen fixation by rhizobia on the roots of legumes.
- Zinc catalyses the formation of growth hormones and promotes the synthesis of RNA and chloroplasts. Thus it is essential for normal growth.
- Chlorine seems to be essential in photosynthesis and is required for plant growth.
- Cobalt is essential for nitrogen fixation by rhizobia and hence aids growth of legumes. However, it is not clear whether it is essential for growth of higher plants.
Preparation of Plant Nutrient Cultures in the Laboratory
Mangaging the Loss of Plant Nutrients from the Soil
- biogas manure – from biogas plants-
- farm yard manure – from wastes of farm animals such as cattle, sheep, goats, poultry, pigs, donkeys, etc;-
- compost manure – from decomposed organic matter; and-
- leguminous green manures, like sunhemp, beans, cowpeas, groundnuts, peas, etc. These young plant materials when ploughed and incorporated into the soil provide organic matter and nitrogen.
This refers to mixed cropping of e.g. cereals and usually leguminous trees like Leucaena leucocephala, which provide nitrogen to the field. The trees take up nutrients from the deeper layers of the soil while the cereals take up their nutrients from the top layers.
Leguminous trees provide the cereals with the humus when their leaves fall and rot on the soil. They also provide forage for animals, and firewood. Agroforestry is also one of the protections against soil erosion.
Manures and Fertilizers
Preparation of Heap and Pit Compost Manure
- Dig compost pit;
- Place dry plant materials. Sprinkle enough water;
- The next layer will be composed of green plant materials or any refuse
- Top this with a mixture of animal manure, soil, and ash;
- Repeat steps 2-4 until the pile reaches a height of 1 m;
- Cover the pit with broad-leaved plants;
- Turn the pile every two weeks. The compost is ready after 3-4 months.
Manures have got several advantages and disadvantages. Some of these are explained below:
- They add nutrients to the soil and at the same time improve soil physical properties such as soil colour, soil structure and water holding capacity of the soil. A soil with good content of organic matter (supplied by manure) holds water and dissolved nutrients efficiently making them available to crop plants.
- Manures supply humus to soil which, in turn, increases the cation exchange capacity of the soil. Humus accounts for 30–90% of the cation exchange capacity of mineral soils. And because of its high cation exchange capacity, humus helps to store nutrient cations, especially ammonium ions, thus reducing the leaching of these nutrients from the soil.
- Manures improve the proliferation of the soil macro- and microorganisms by supplying the nutrients and conducive conditions needed by these organisms for survival. These organisms play a vital role in soil fertility and plant nutrition by decomposing organic matter which releases nutrients to the
- Manures provide organic matter which acts as the binding materials for soil particles, making them more compact and hence resistant to the impact of rain drops and surface run off of water. Thus, it reduces soil erosion.
- Manures can remain in the soil for a long time and they can provide the nutrients to crops for several growing seasons. Its nutrients are released slowly over a long period of time
- They do not change the soil pH greatly as the inorganic fertilizers do.
- Humus from organic matter is dark in colour and imparts this black colouration to the soil. Black colour absorbs more heat and hence helps to regulate soil temperature.
- Manures contain and provide little nutrients per unit volume and weight. One has to apply tremendous amounts of manure to meet the requirements of plants.
- Their bulkiness and volume makes it difficult to store, handle or apply in the field. It requires more space to store or transport and more labour to apply manures in the field.
- Some kinds of manures e.g. sludge or industrial and municipal wastes may contain toxic chemicals which can harm humans, animals or soils to which it is applied.
- Manures act very slowly in that they release nutrients to the soil at a very slow rate.
- If the plant materials used to make manure is infested with plant pests or weed seeds; or infected with diseases, there is a risk of spreading them to the farm.
- Manures easily lose nutrients if stored improperly. Under hot conditions, manures produce a lot of heat that leads to loss of136nitrogen through vapourization. Soluble nutrients are easily leached
Types of Synthetic Fertilisers Used in Tanzania
- Sulphate of ammonia, (NH4)SO4;
- Calcium Ammonium Nitrate, CAN;
- NPK;
- Superphosphates;
- Ammonium chloride, NH4Cl;
- Urea, CO(NH2)2
- Ammonium nitrate, NH4NO3;
- Potassium sulphate, K2SO4
- Potassium chloride, KCl; etc.
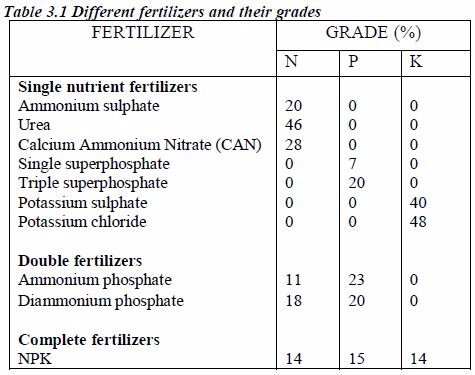
The Concept of Fertiliser Grades and Analysis
- Single nutrient fertilizer or straight fertilizers: These are fertilizers containing only one of the primary nutrient elements.
- Double nutrient fertilizer: These contain two primary nutrient elements.
- Complete fertilizers or complex fertilizers: These are materials that contain all three primary elements, N, P and K.
Types of fertilizers

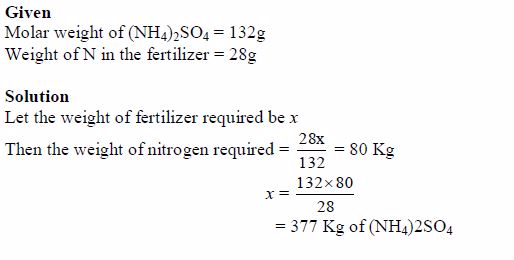
- In soils whose fertility status is extremely low;
- In closely spaced crops such as rice, wheat, pasture, millet, etc;
- If the fertilizer used is in fine particles (granules or powdered); and
- If the field is properly prepared and is in good tilth.
- where the amount of fertilizer to apply is limited;
- for widely-spaced crops;
- when small labour is required ; and
- in seedbed preparation.
Explain the advantages and disadvantages of artificial fertilizers as compared to natural manures
- They contain more nutrients per unit volume and weight.
- They are compact and hence easy to transport, store and apply to the field as compared to manures which are bulky in nature.
- They contain specific quantities of plant nutrients per unit volume and weight. So the quantity of a particular nutrient to be applied to the soil can easily be estimated to avoid overapplication. For example the weight of nitrogen in a kilogram of NH4NO3 can easily be established and quantified.
- They dissolve quickly and hence provide nutrients to plants instantly as they are added to the soil.
- They are used only for one growing season as they are short-lived. Because the uptake of the nutrients in the fertilizer is very high, no or few nutrients would have remained in the soil in the next growing season.
- Some acidic mineral fertilizers such as NH4Cl and (NH4)2SO4 contribute to soil acidity. When these fertilizers are applied to the soil repeatedly, they can make the soil acidic and hence not fit for plant growth.
- Prolonged use of artificial fertilisers may lead to deterioration of the soil structure and poisoning of soil and soil microbes.
- Extensive use of fertilizers may cause contamination of drinking water resources, by especially nitrate fertilizers. Nitrates dissolved in water are not removed by normal purification processes. In the body nitrates may be converted into nitrosamines. These are carcinogenic (cancer-causing) compounds
- If too much nitrogenous fertilizer is used, serious pollution can occur. One kind of pollution is called eutrophication. Excess of the fertilizer applied finally finds its way to water. This encourages fast growth and huge increase in the number of microscopic, aquatic plants called algae, a phenomenon called algal bloom. Proliferation of algae on the water surface blocks sunlight from reaching the plants beneath the water. These plants can not carry out photosynthesis and, therefore, they die. Bacteria and other decomposers feed on these dead plants and increase in number. These decomposers use up all the oxygen dissolved in the water. Without ample supply of oxygen fish and other organisms living in the water die.
- They can scorch (burn) and kill crop plants if not applied under manufacture‟s directions or even harm humans if not properly handled.
- Fertilizers are expensive to purchase and hence not affordable.
Soil Fertility and Productivity
The Concept of Soil Fertility and Soil Productivity
- Chemical soil fertility: this is the fertility due to chemical processes that contribute to soil fertility. The chemical soil fertility falls under two categories namely,(i) potential chemical fertility, due to cations in soil solution; and(ii) active chemical fertility, which is due to exchangeable cations adsorbed to the soil colloidal surface or negatively charged plant roots.
- Physical soil fertility: the fertility contributed by soil moisture, texture, structure, temperature, etc.
- Biological soil fertility: this is due to organic matter content, and soil microorganisms.
- Soil fertility. This refers to the ability of the soil to supply the essential plant nutrients required for maximum plant growth.
- Plant factors. These includes yield potential, root growth characteristic and genetic make up of a particular crop plant. This means that some crop plants are high-yielding than other plants of the same species and are thus likely to give more crop yields. Also plants with good root development are likely to absorb more nutrients from the soil, grow better and give good yield as compared to plants with poor root development. Genetic make up of a plant also plays a vital role in this respect. For example hybrid maize will always survive harsh146soil and environment conditions than local varieties of maize and, therefore, will give high yields.
- Environment factors. These factors include climatic factors and agronomic practices.Climatic factors – These are factors such as temperature, precipitation (rainfall), radiation, humidity, altitude, etc.Agronomic practices include weed control, pest and disease control, good soil preparation, plant population, etc. Yield can be measured in terms of grain yield, tubers yield, dry matter, height of plants, number of leaves, number and size of fruits, berries, etc.
- It has adequate water retention capacity;
- It is well aerated; and
- It is able to supply adequate amounts of the nutrients to plant.
Difference between Soil Fertility and Soil Productivity
The texture and structure of the soil
The depth of the soil profile
The Causes of Loss in Soil Fertility
- Soil erosion: Most plant nutrients are contained in top and sub soil. It is these nutrients that are available to plants for growth and development. When the top player of the soil is removed by erosion, the nutrients in it are lost too. Erosion agents tend to clear and transport the soil from its original site to another site far away. By so doing, the nutrient elements that are contained in this soil are also carried together with the soil. This process then leads to loss of nutrients from the soil and hence loss in soil fertility.
- Leaching: This refers to the flushing of plant nutrients from the top to the lower layers of the soil and beyond the reach of plant150roots. This is caused by heavy rainfall or flood irrigation. The process makes the nutrients unavailable to plants as it washes them for beyond the root zone.
- Monoculture: The cultivation of the same type of crop on a piece of land year after year, leads to soil depletion if manures or fertilizers are not added. Different plants have specific needs for particular mineral compounds. If the same type of crop is grown on as similar field continuously over a number of years, then the soil will become deficient of the minerals taken up by that crop.
- Denitrification: Denitrifying bacteria convert nitrates of the soil to gaseous nitrogen which escapes to the atmosphere, thus depriving the soil of nitrogen. However, this process is counteracted by another type of beneficial bacteria called nitrifying bacteria, which again convert nitrogen of the air back to soil nitrates by a process called nitrification.
- Nutrient uptake by plants: Growing plants absorb nutrients from the soil for growth and development. If the nutrients taken up by plants are not replaced through adding manure or inorganic fertilizers, the soil becomes deficient of these minerals.
- Volatilization: This refers to the conversion of ammonium compounds into ammonia gas. Nitrogenous compounds in the soil are decomposed by heat into ammonia gas. Also when nitrogenous fertilizers are added to highly basic soils, they react with sodium hydroxide in the soil to release ammonia gas which simply escapes to the atmosphere.NH4+(aq) + OH–(aq) → NH3(g) + H2O(l)
- Accumulation of salts: Under normal conditions, rain water washes the mineral salts away, thereby keeping their concentrations in the soil low. However in arid and semi arid151regions the salts accumulate in the soil as the rain falling there is irregular and is insufficient to wash away the salts. This, together with the high evaporation rate and poor drainage, leads to excessive accumulation of salts on or below the soil surface.
- Change in soil pH: Use of acidic fertilizers over a long period of time can make the soil acidic. Change in soil pH affects the activity of soil microorganisms and availability of some plant nutrients. This, in turn, affects the fertility of the soil.
- Burning of vegetation: Burning crop residues and vegetation deprives the soil microorganisms of the organic matter they require for survival and proliferation. This affects microbial activities such as nitrogen fixation and decomposition of organic matter






























Leave a Reply