Topic 2- Organic Chemistry – Chemistry Form Four
Introduction to Organic Chemistry
Difference between Organic from Inorganic Chemistry
Distinguish organic from inorganic chemistry
The Importance of Organic Chemistry in Life
Explain the importance of organic chemistry in life
- some are used as fuel or converted into fuels;
- some are used for making detergents, dyes, drugs, paints, and cosmetics; and yet some are used for making polyethene, polyvinyl chloride (PVC) and other plastics.
The Origin of Organic Compounds
Crude oil is one of the world’s major natural resources. The oil is the result of a process that began up to 400 million years ago. Prehistoric marine organisms died and sunk to the sea bed and were covered by mud and sand. The change into crude oil and natural gas was brought about by high pressure, high temperature and bacteria acting over millions of years. The original organic material broke down into hydrocarbons. Compression of the mud above the hydrocarbon mixture transformed it into shale.
Then geological movements and pressure changed this shale into harder rocks, squeezing out the oil and gas. The oil and gas moved upwards through the porous rocks, moving from high- pressure to low-pressure conditions. Sometimes they reached the surface, but often they became trapped by a layer of non porous rock. Reservoirs of oil and gas were created. These reservoirs are not lakes of oil or pockets of gas. Instead, the oil or gas is spreadthroughout the pores in coarse rocks such as sandstone or limestone the same as water is held in a sponge.
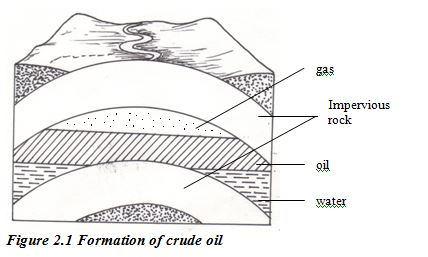
The Fractional Distillation of Crude Oil
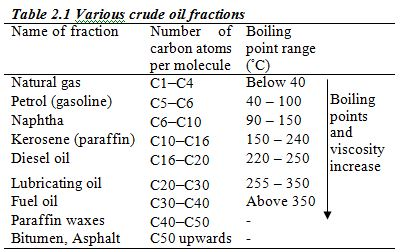
Crude oil from an oilfield is separated from impurities such as sand and water and is pumped through pipelines to the refinery. At the refinery, fractional distillation is used to separate the crude oil into several fractions, each fraction containing several hydrocarbons which boil within a certain range of temperatures. These different boiling points are roughly related to the number of carbon atoms in the hydrocarbon (Table 2.1)
After fractional distillation, impurities are removed. The commonest impurity is sulphur, which is removed and used to manufacture sulphuric acid. If petrol (gasoline) containing sulphur is not purified before it is used in an internal combustion engine, the exhaust fumes will contain oxides of sulphur (SO2 and SO3). These are poisonous gases and will pollute the environment.

Uses of different petroleum fractions
The three Families of Hydrocarbons
Alkanes
Table 2.2 Formulae and physical properties of some alkanes
| name | Molecular formula | Melting point (oC) | Boiling point (oC) | Density (gcm–3) |
| Methane | CH4 | –183 | –162 | gas |
| Ethane | C2H6 | –172 | –89 | gas |
| Propane | C3H8 | –188 | –42 | gas |
| Butane | C4H10 | –135 | –1 | gas |
| Pentane | C5H12 | –130 | 36 | 0.626 |
| Hexane | C6H14 | –95 | 69 | 0.659 |
| Heptane | C7H16 | –91 | 98 | 0.684 |
| Octane | C8H18 | –57 | 126 | 0.703 |
| Nonane | C9H20 | –54 | 151 | 0.718 |
| Decane | C10H22 | –30 | 174 | 0.730 |
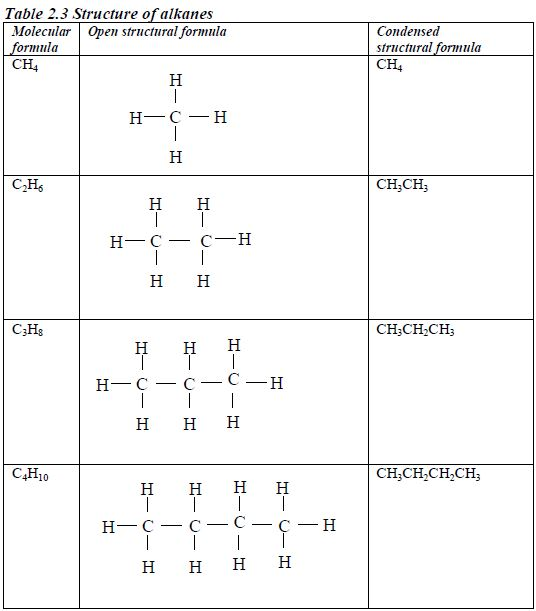
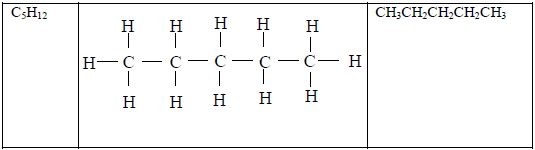
The Homologous Series of the Three Families of Hydrocarbons
- All members obey the general molecular formula, e.g. for alkanes Cn H2n+2, alkenes Cn H2n and alkynes, Cn H2n-2.
- Each member differs, in molecular formula, from the next by CH2, e.g. alkanes are CH4, C2H6, C3H8, and so on.
- All members show similar chemical properties.
- The general properties of members change gradually in the same direction along the series, e.g. in alkanes, boiling points and freezing points rise with increase in the number of carbon atoms (CH4 – a gas; C5H12 – a liquid; C20H42 – a solid at ordinary temperatures and pressure).
- General methods of preparation are known which can be applied to any member of the series. Other homologous series are alcohols, CnH2n+1 and carboxylic acids, CnH2n+1COOH.
Nomenclature of alkanes
Rules of Nomenclature
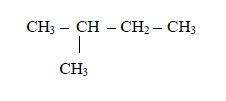
- CH3 – methyl
- CH3CH2 – ethyl
- CH3CH2CH2 – propyl
- Cl – chloro
- Br – bromo
- I – iodo
- F – flouro
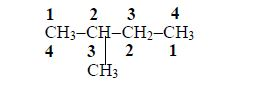
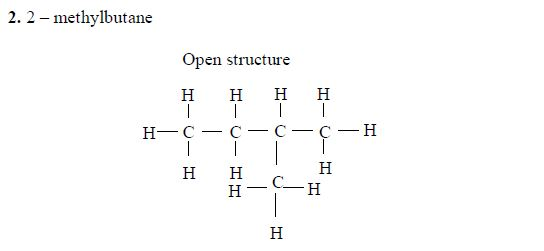
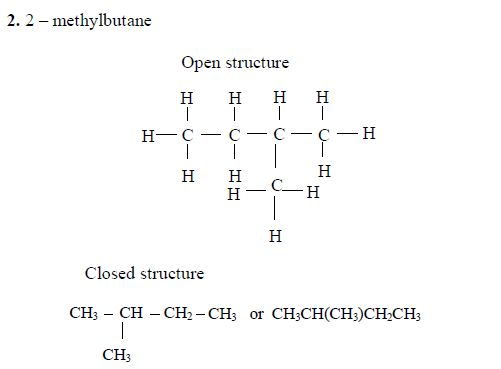
- 2 – methylbutane
- 3 – methylbutane
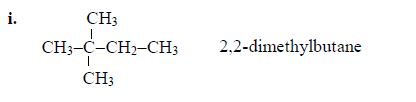
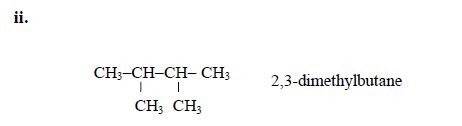
The Concept of Isomerism
Explain the concept of isomerism
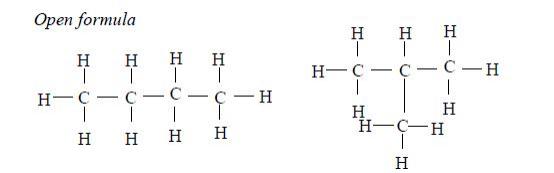

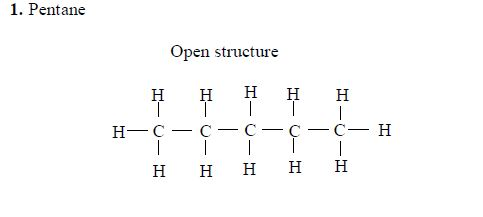
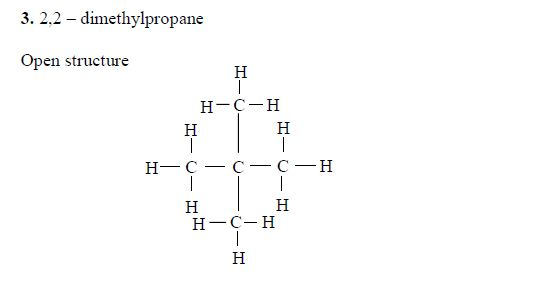
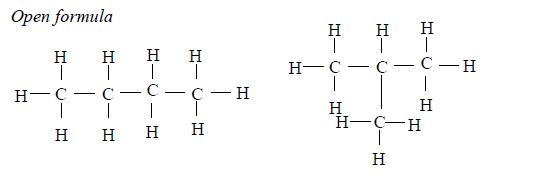
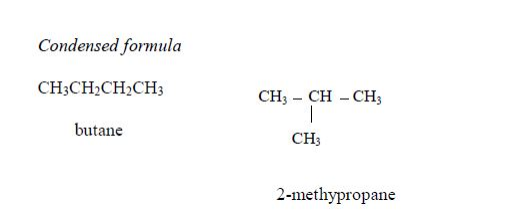
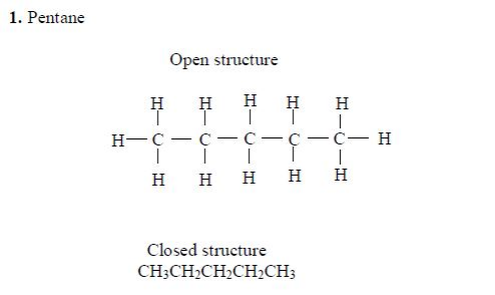
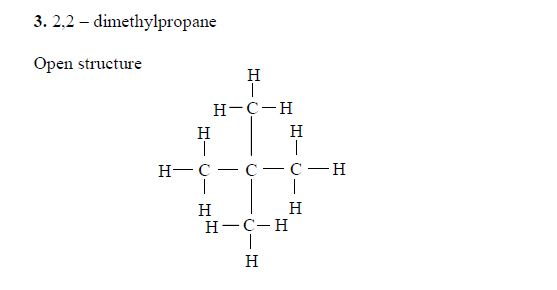
Isomers of Hydrocarbons up to Five Carbon Atoms
alkanes showing isomerism

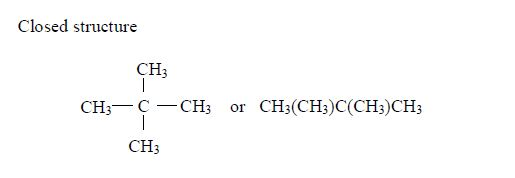
Alkenes
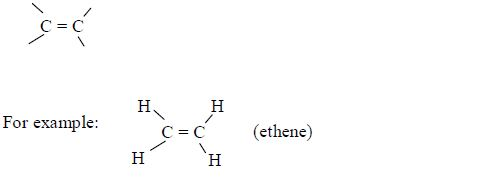
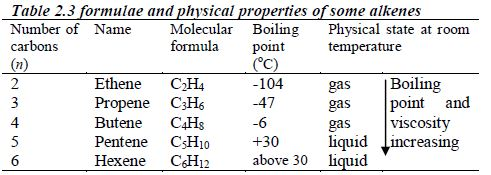
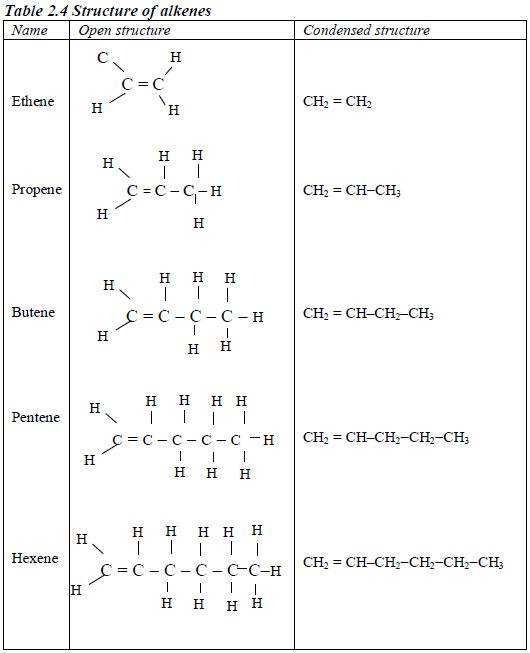
- Names of alkenes have the ending –ene.
- The double bond is given the number of the carbon atom where it begins.
- In addition to the rules discussed earlier, the position of the double bond must be included in the name .Examples: CH3–CH=CH3 prop-1-ene , CH3–CH=CH–CH3 but-2-ene
- Double bonds are given the lowest number possible, usually lower than the functional groups.
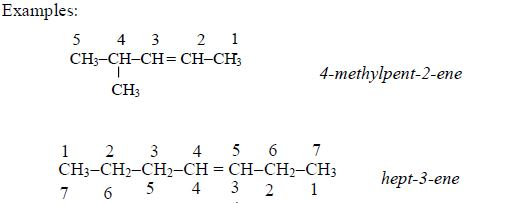
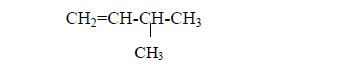
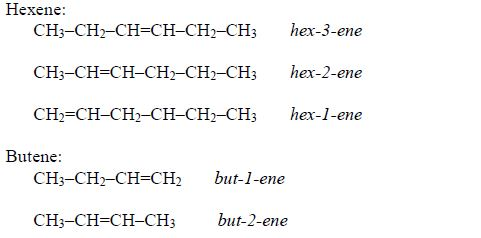
Alkynes
These are homologous series of hydrocarbons with the general formula CnH2n-2. The common structural feature is the presence of a carbon-carbon triple bond (CC). There is no member for n = 1 because, as for the alkenes, there needs to be at least two carbonatoms present to have a triple bond. Table 2.5 shows the first five members of the alkynes.
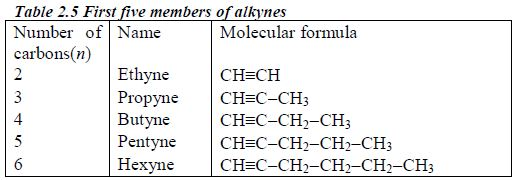
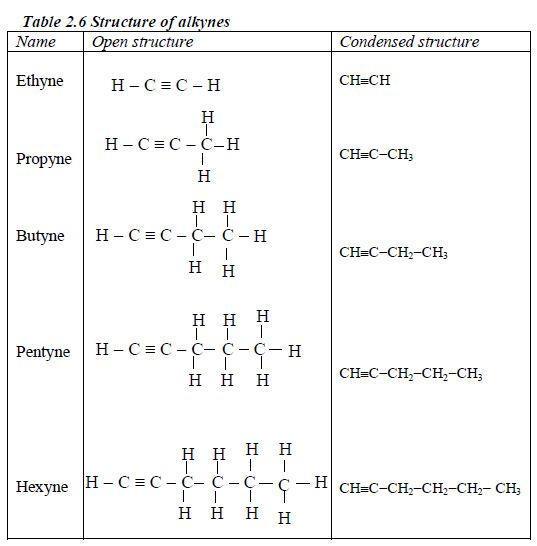
Nomenclature of alkynes
- Alkynes have the ending –yne
- Triple bond is given the lowest number possible, usually lower than the functional groups.
- As for the alkenes the position of the triple bond must be included in the name.
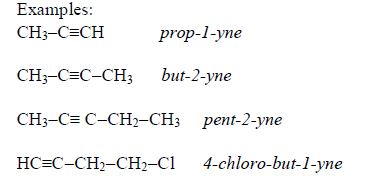
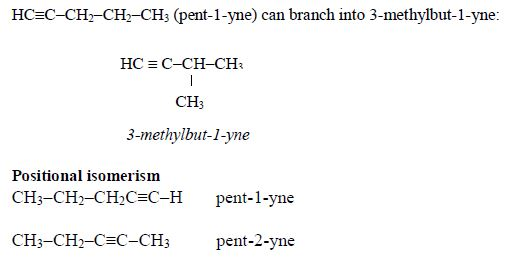
- The first four alkanes are gases at room temperature, the next twelve (C5–C17) are liquids, and the rest are solids.
- The boiling and melting points of unbranched alkanes increase as the molar masses increase. The larger the molecule, the higher the boiling point or melting point.
- Branched alkanes have lower boiling points than the unbranched alkanes,
- Alkanes are very sparingly soluble in water but they easily dissolve in organic solvents.
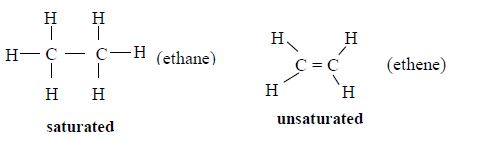
- In an alkane molecule, each carbon atom forms four single covalent bonds. This means that alkanes are saturated hydrocarbons.
- They are insoluble in water but soluble in organic solvents suchas benzene, ether and chloroform.
- They are less dense than water.
- The boiling point increases with increase in molecular weight i.e., increase in the number of carbon atoms.
The Chemical Properties of Alkanes, Alkenes and Alkynes
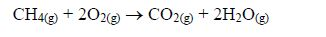
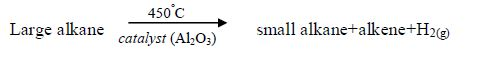
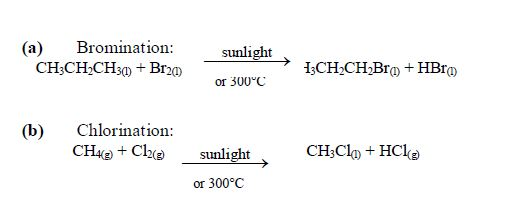
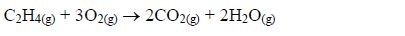
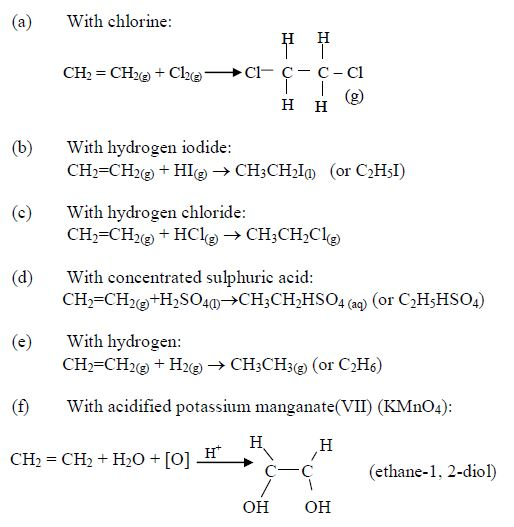
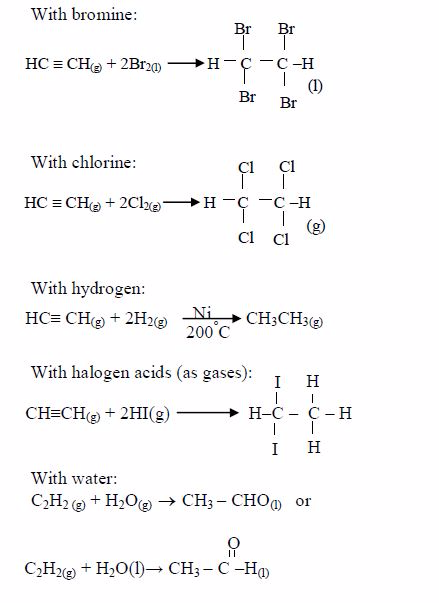
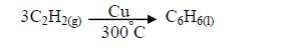
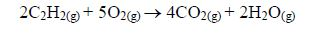
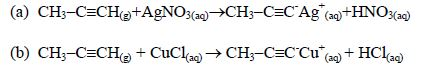
This is a test for terminal alkynes, i.e. those with C≡C at the end. For those with C≡C at the centre, no reactions take place e.g. CH3–CH2–C≡C—CH2–CH3 + AgNO3→ No reaction!
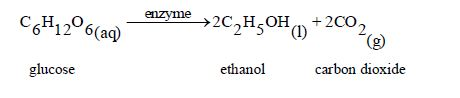
Preparation of Ethanol in the Laboratory
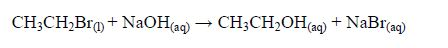
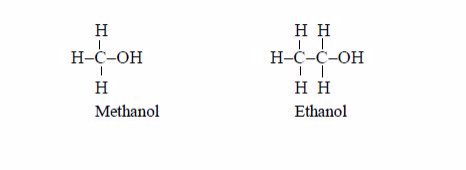
The Homology of Alcohols up to Five Carbon Atoms
Write the homology of alcohols up to five carbon atoms
Other members of the series are as shown below:
| Number of carbons per molecule (n) | Name | Formula |
| 1 | Methanol | CH3OH |
| 2 | Ethanol | C2H5OH |
| 3 | Propanol | C3H7OH |
| 4 | Butanol | C4H9OH |
| 5 | Pentanol | C5H11OH |
| 6 | Hexanol | C6H13OH |
| 7 | Heptanol | C7H15OH |
| 8 | Octanol | C8H17OH |
| 9 | Nonanol | C9H19OH |
| 10 | Decanol | C10H21OH |
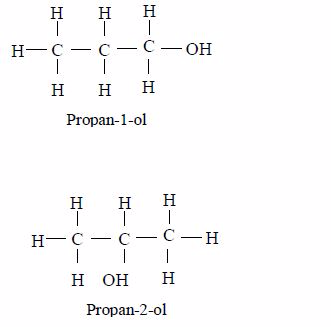
Write structure of all isomers of saturated alcohols up to five carbon atoms
- For branched alcohols, the–OH group is given the lowest number than the alkyl group.
- The alcohol group (–OH) or the alkyl groups can be attached to different positions of the carbon chain. It is the different positions of these groups that result to the different names of the alcohol.
- The carbon chain may have several branches of different alkyl groups.
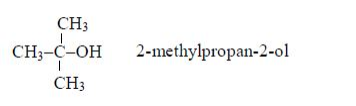
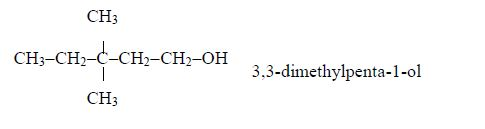
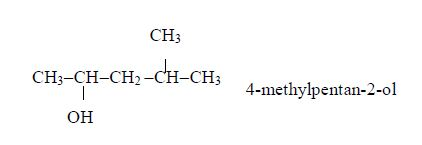
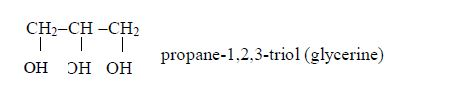
Isomers of Alcohols up to Five Carbon Atoms
Name all isomers of alcohols up to five carbon atoms

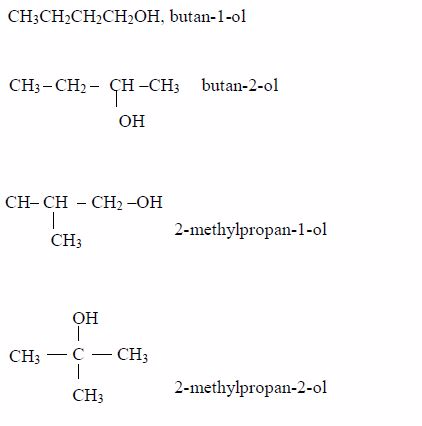
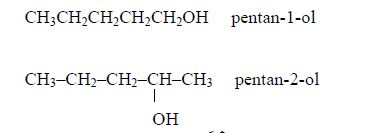

The Properties of Alcohol
- It is a clear, colourless liquid with a boiling point of 78°C.
- It is readily soluble in water and it mixes completely with it (miscible).
- It evaporates very easily when exposed to air (it is a very volatile liquid).
Chemical properties of ethanol
Reaction with oxygen (combustion)
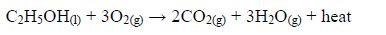
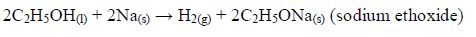
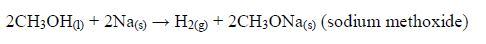
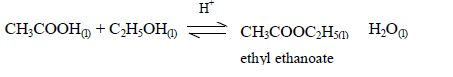
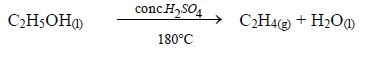
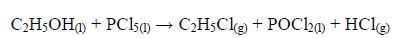
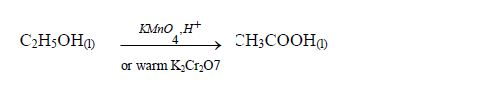
- Ethanol burns with a clear flame, giving out quite a lot of heat.C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g) + heat On a small scale, ethanol can be used as methylated spirit (ethanol mixed with methanol or other compounds) in spirit lamps and stoves.
- Ethanol produced by fermentation of sugar from sugar cane has been used as an alternative fuel to gasoline (petrol), or mixed with gasoline to produce “gasohol”.
The Harmful Effects of Alcohol
Explain the harmful effects of alcohols
- Even just one drink impairs coordination and judgement. It is a factor among the many causes of road accident as it leads to blurred vision.
- It leads to lack of muscular control (e.g. drunken stagger) and ultimately to coma, the state in which a person is said to be dead–drunk.
- Prolonged consumption of too much alcohol causes liver deterioration (cirrhosis of the liver) which can cause liver failure and death. Also heavy drinking eventually damages the muscle tissue of the heart. There may well be some long–term damage to the brain. All these health effects accelerate death.
- Alcohol is a depressive drug and can be addictive. Occasional drinking may lead to alcohol addiction, a condition during which a person is said to be alcoholic. When a drunkard finds him/herself in such a condition it is very difficult to go without drinking. This can eventually lead to poverty as a drunkard spends most of his/her time and money drinking (buzzing).
- Alcohol can make someone aggressive. This accounts for many arrests, conviction and jail sentences.
- Excessive drinking causes depression and other mental disorders.
- It can lead to gastric ulcers, high blood pressure and cancer of the mouth, throat, and gullet. People who smoke as well are at greater risk from thesecancers.
Carboxylic Acids
- milk (lactic acid)
- citrus fruits (citric acid);
- tobacco (nicotinic acid); and
- tea (tartaric acid).
The Oxidation of Ethanol to Ethanoic Acid
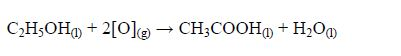
The Structures of the Homologues of Carboxylic Acids up to Five Carbon Atoms

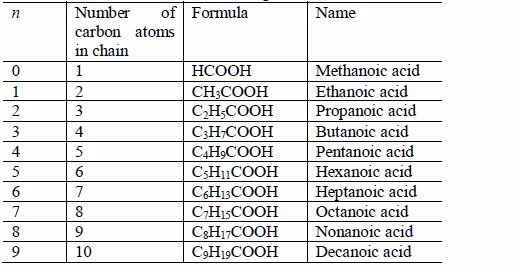
The Isomers of Carboxylic Acids up to Five Carbon Atoms
Like other organic compounds, carboxylic acids also exhibit isomerism. Isomers of carboxylic acid are a result of branching of the hydrocarbon end (R) rather than the position of the carboxyl group in a molecule of the carboxylic acid. More isomers of the carboxylic acids can be created by branching the hydrocarbon end in as many different ways as possible.
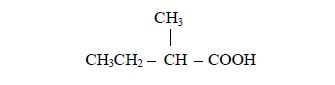
- the alkyl group is methyl;
- it is attached to carbon number and
- the acid to which it is attached is butanoic acid,CH3CH2CH2COOH
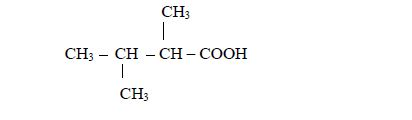
- there are two methyl groups, one attached to carbon number 2 and the other to carbon number 3; and
- they are both attached to butanoic acid chain.
Isomerism and nomenclature
- Butanoic acid, C3H7COOH or C3H7CO2H or CH3CH2CH2COOH
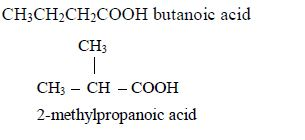
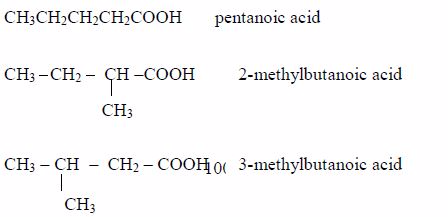
- Pentanoic acid, C4H9COOH
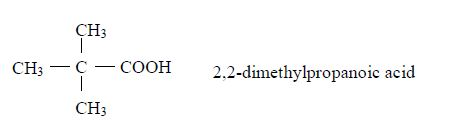
The Properties of Carboxylic Acids
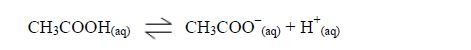

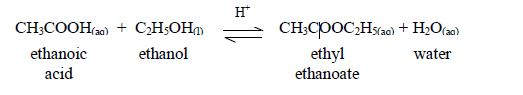
Esters are manufactured for use as solvents, food flavourings, and fragrance for perfumes and beauty products. Ethyl ethanoate is just one example of many esters. The esters usually have strong and pleasant smells. Many of these compounds occur naturally.
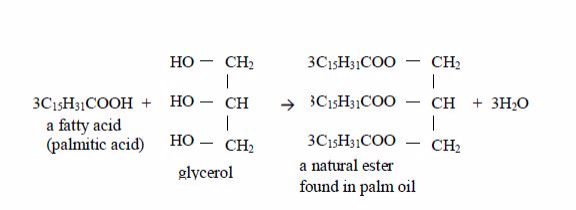
They are responsible for the flavours in fruits and for the scents of flowers. Fats and oils are naturally occurring esters used for energy storage in plants and animals. Some of the naturally occurring esters include:
- vegetable oils e.g. palm oil, groundnut oil, cashewnut oil, olive oil, sunflower oil, etc; and
- animal fats.

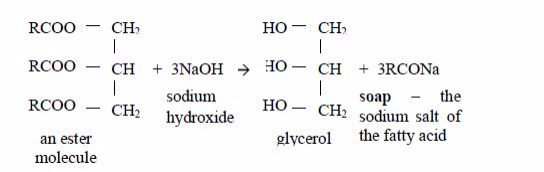
Preparation of Soap from Animal Fats or Vegetable Oil
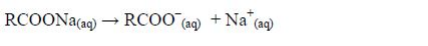































Leave a Reply