TOPIC 5: THERMAL EXPANSION – PHYSICS NOTES FORM THREE
Thermal Energy
The Concept of Heat
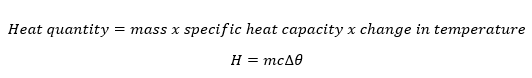
Source of Thermal Energy in Everyday Life
Difference between Heat and Temperature
When you heat a substance, either of two things can happen: the temperature of the substance can rise or the state of substance can change.
| Heat | Temperature | |
|---|---|---|
| Definition | Heat is energy that is transferred from one body to another as the result of a difference in temperature. | Temperature is a measure of hotness or coldness expressed in terms of any of several arbitrary scales like Celsius and Fahrenheit. |
| Symbol | Q | T |
| Unit | Joules | Kelvin, Celsius or Fahrenheit |
| SI unit | Joule | Kelvin |
| Particles | Heat is a measure of how many atoms there are in a substance multiplied by how much energy each atom possesses. | Temperature is related to how fast the atoms within a substance are moving. The ‘temperature’ of an object is like the water level – it determines the direction in which ‘heat’ will flow. |
| Ability to do work | Heat has the ability to do work. | Temperature can only be used to measure the degree of hea |
- Its temperature may rise
- Its state may change
- It may expand
DEMONSTRATION OF EXPANSION OF SOLIDS
- The ball and Ring Experiment
- The Bar and Gap Experiment


Expansion of Solids in terms of Kinetic Theory of Matter
Normally Expansion and contraction is accompanied by tremendous forces; The presence of force indicates the expansion and contraction is resisted.

- Consists a strong metal blocker with a pair of vertical jaws J and a strong metal Bar R. The metal bar has a wing nut N at one end and an eye at the other end.
- The bar is placed between the two pair of Jaws
- A short cast iron C is inserted in the eye of the bar.
- The bar is then heated it expands and the wing not screwed to tighten the bar R against the jaws.
- The bar is then allowed to cool as it cools, it contracts the short cast Iron rod C which presses against the jaws of the bar breaker, Resists the contraction of the Metal bar R.
- The resistance to the contraction of the bar sets up very large forces which breaks the short cast Iron rod C in the eye of metal bar, Because the contracting bar is trying to pull itself through the small gap in the frame.
Expansivity of Different Solids
- The length of the solid
- The temperature to which the solid is heated
- The nature of solid
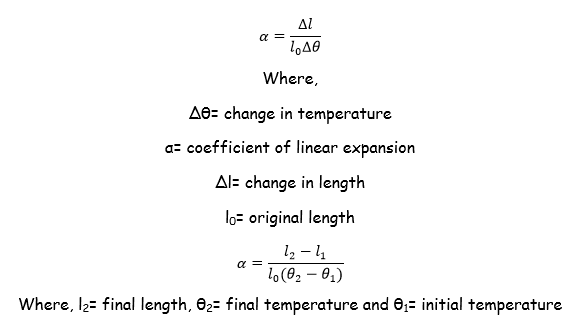
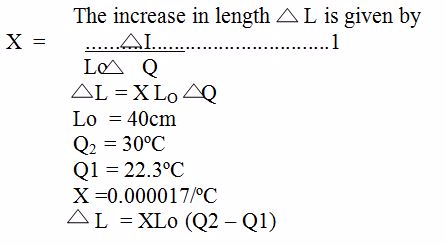
Table which shows the coefficient of Expansivity Constant.
| Substance | Linear Expasivity (PerºC) |
| Aluminum (Al) | 0.000026 |
| Brass | 0.000019 |
| Copper | 0.000017 |
| Iron | 0.000012 |
| Steel | 0.000012 |
| Concrete | 0.000011 |
| Glass | 0.0000085 |
| Invar ( Alloy of Iron and Nickel) | 0.000001 |
The Application of Expansion of Solids in Daily Life
Thermal Expansion of Liquids
The Apparent Expansion of Liquids
Demonstrate the Effects of Heat on Liquids
Activity 1
- Large heat safe glass bowl
- Cooking Oil
- Food Coloring
- Two 2×4 blocks
- Candle
- Match or Lighter
- Begin by filling a large glass bowl with cooking oil.
- Next, add between 5-10 drops of food coloring into the oil. Helpful Tip: Place the drops near the center of the bowl.
- Prop the bowl up off the table using two 2×4 blocks.
- Light a candle and carefully place it under the bowl. The flame of the candle should touch the bottom of the glass bowl.
- Look through the side of the glass bowl and watch carefully to observe what happens. Helpful Tip: It will likely take 5 minutes before you see anything happen to the liquid/food coloring.
You can alternatively follow the experiment through the following vedio
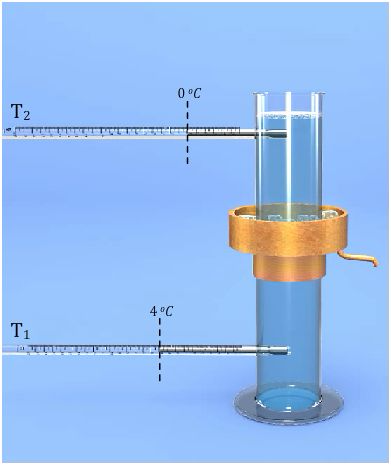
Verification of Anomalous of Water
In the year 1805, the scientist T. C. Hope devised a simple arrangement, known as Hope’s apparatus, to demonstrate the anomalous behaviour of water.
The Application of Expansion of Liquids in Everyday Life
Thermal Expansion of Gases
The Concept of Thermal Expansion of Gases
The Relationship between Volume and Temperature of Fixed Mass of Air at Constant Pressure
The Relationship between Pressure and Volume of a Fixed Mass of Air at Constant Temperature
The Relationship between Pressure and Temperature of a Fixed Mass of Air at Constant Volume
The General Gas Equation from the Gas Laws
- pV = constant (when T is kept constant)
- V/T = constant (when p is kept constant)
- P/T= constant (when V is kept constant)
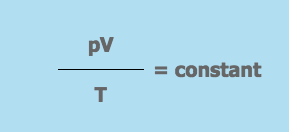
- p = the pressure of the gas
- V = the volume the gas occupies
- T = the gas temperature on the Kelvin scale
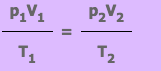
Absolute Scale of Temperature
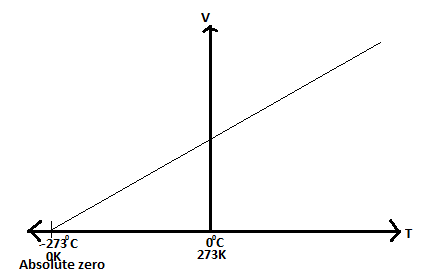
Convertion of Temperature in Degrees Centigrade (Celsius) to Kelvin
- Temperature in °C + 273 = Temperature in K
- Temperature in K – 273 = Temperature in °C
Standard Temperature and Pressure (S.T.P)
Expansion of Gas in Daily Life
































Leave a Reply