Topic 6- Ionic Theory and Electrolysis – Chemistry Form Three
Ionic Theory
Electrolytes and Non-electrolytes
Electrolysis: decomposition of a compound in solution or molten state by passing electricity through it.
Conductor: a solid substance that allows electricity to pass through it. All metals are included in this class.
Non-conductor or insulator: a solid substance that does not allow electricity to flow through it. All non-metals fall in this class.
Electrolyte: a substance which, when dissolved or molten, conducts electricity and is decomposed by it.
Non-electrolyte: a compound which cannot conduct electricity, be it in molten or solution state.
Electrode: a graphite or metal pole (rod) or plate through which the electric current enters or leaves the electrolyte.
Cathode: a negative electrode which leads electrons into the electrolyte.
Anode: a positive electrode which leads electrons out of the electrolyte.
Ion: a positively or negatively charged atom or radical (group of atoms).
Cation: a positive ion which moves to the cathode during electrolysis.
Anion: a negative ion which moves to the anode during electrolysis.
Electrolytes and non-electrolytes
The Mechanisms of Electrolysis
Set up electrolytic cells of different electrolytes in the molten and aqueous states
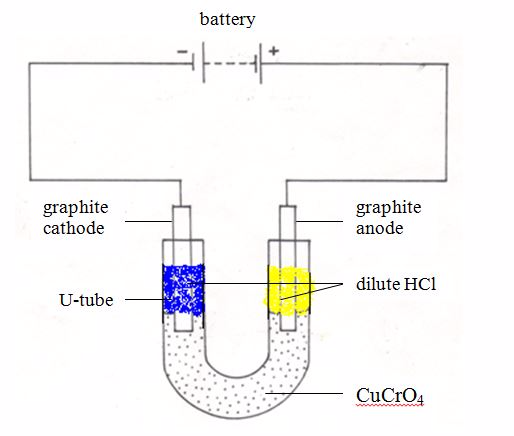
Ionic Migrations During Electrolysis and the Preferential Discharge of Ions at the Electrodes
When a salt such as sodium chloride is dissolved in water, its ions are set free to move. So the solution can be electrolysed. Since the salts, alkalis and acids are dissolved in water, most of the solutions are aqueous. There is then a complication in electrolysis of such substances in aqueous form. This is because the water used to dissolve them also do ionize partially (it is a weak electrolyte)
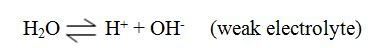
During electrolysis, Cu2+ and H+ ions move to the cathode while SO42-and OH– ions move to the anode.
| Cathode | Anode |
| Cu2+ | SO42- |
| H+ | OH– |
- the position of the metal ion or radical in the electrochemical series;
- the concentration or nature of the ions (or electrolyte) to be discharged; and
- the nature of the electrodes used.
The position of ion or radical in the electrochemical series
| Cations | Anions | ||
| K+ | ease of dischargeincreases | SO42- | ease of discharge increases |
| Ca2+ | NO3– | ||
| Na+ | Cl– | ||
| Mg2+ | Br– | ||
| Al3+ | I– | ||
| Zn2+ | OH– | ||
| Fe2+ | |||
| Pb2+ | |||
| H+ | |||
| Cu2+ | |||
| Ag+ |
The arrangement of ions above is the same as that of the electrochemical series. If all other factors are constant, any ion will be discharged from solution in preference to those above it.
| Cathode | Anode | |
| Cu2+ | SO42- | |
| H+ | OH– | |
| Cu2+ + 2e– → Cu(s)copper is discharged (loses its charge) | 4OH–→2H2O(l)+O2(g)+4e– hydroxyl ion is discharged |
- At cathode, copper is deposited
- At anode, oxygen is given out (liberated)
- The solution at the end of electrolysis is colourless and acidic because in the electrolyte there are left H+ and SO42- ions, which remain moving freely in the solution as ionic sulphuric acid.
- aqueous solution;
- concentrated aqueous solution; or
- fused or molten state.
| Cathode | Anode |
| Na+and H+ | Cl–and OH– |
| H+ions are discharged in preference to Na+ions: 2H+ + 2e– → H2(g) | OH–ions are discharged in preference to Cl–ions:4OH–→2H2O(l) +O2(g)+4e– |
- At cathode, hydrogen is given out
- At anode, oxygen is out
- The concentration of the solution remains constant since all Na+ and Cl– ions remain undisturbed in the solution. This means that the Na+ and Cl– ions that are left in the solution are equivalent to sodium chloride solution.
| Cathode | Anode |
| Na+and H+H+ ions are discharged in preference to Na+ ions since Na+ and H+ are very far from each other in e. c.s2H+ + 2e– → H2(g) | Cl–and OH–Cl–ions are discharged in preference to OH– ions since Cl– and OH– ions are very close to each other in the e. c. s (and because there are more Cl– ions in the solution) 2Cl–→ Cl2(g) + 2e– |
- At cathode H2(g) is given off
- At anode Cl2(g) is given off
- The solution becomes progressively more alkaline as the electrolysis goes on because Na+and OH–ions remain in solution as caustic soda (sodium hydroxide) solution. This is the main method used in the manufacture of sodium hydroxide in industry. Na+(aq) + OH–(aq) → NaOH(aq)
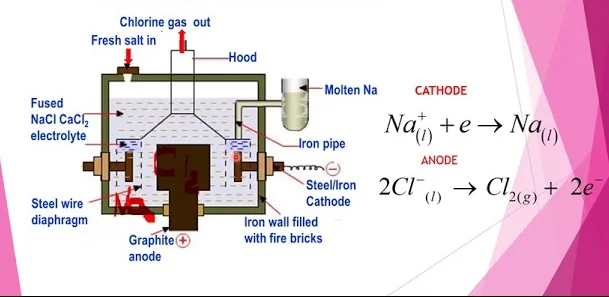
| Cathode | Anode |
| Na+ + e– → Na(l) | 2Cl– → Cl2(g) + 2e– |
- At cathode sodium is liberated (deposited)
- At anode chlorine is given off
| Cathode | Anode |
| 2H+ + 2e– → H2(g) | SO42- and OH– 4OH–→2H2O(l)+O2(g)+4e– |
- At cathode H2(g) is given out
- At anode O2(g) is given out
- The solution becomes acidic at the end of electrolysis because of the acidic ions (SO42-) left in the finala solution
Charge flow during electrolysis
The coulomb is the electrolytic unit of charge. A current of one ampere is the rate of flow of charge equal to one coulomb per second.The charge is calculated from the knowledge of the number of seconds for which a steady current is passed.
| Current in circuit | Time taken | Total charge |
| 1 ampere | 1 second | 1 coulomb |
| 1 ampere | 10 seconds | 10 coulombs |
| 20 amperes | 10 seconds | 200 coulombs |
| A amperes | t seconds | At coulombs |
Flow of charge required to liberate 1 mole of element during electrolysis
The number of faradays (moles of electrons) required to liberate 1 mole of an element during electrolysis is deduced from the equation for the electrode reaction.
| Element | Electrode reaction | Faradays |
|
Na+ + e– → Na(s) | 1 |
|
Cu2+ + 2e– → Cu(s) | 2 |
|
Al3+ + 3e– → Al(s) | 3 |
|
2Cl– → Cl2(g) + 2e– | 2 |
- The mass of an element liberated by 1 coulomb of electricity during electrolysis is called electrochemical equivalent of that element.
- The mass of an element deposited or liberated by 1 faraday during electrolysis is called chemicale quivalent of that element.
Laws of Electrolysis
- the time of passing the steady current;
- the magnitude of the steady current passed; and
- the charge on the ion of the element.
An Experiment to Verify Faraday’s First Law of Electrolysis
Specimen results for electrolysis of copper (II) sulphate
| Current (amps) | Time(s) | Quantity of electricity (coulombs) | Mass of copper deposited (grams) |
| 0.21 | 15 × 60=900 | 900 ×0.21=189 | 0.063 |
| 0.21 | 30 × 60=1800 | 1800 ×0.21=378 | 0.129 |
| 0.21 | 45 × 60=2700 | 2700 ×0.21=576 | 0.187 |
| 0.21 | 60 × 60=3600 | 3600 × 0.21=756 | 0.250 |
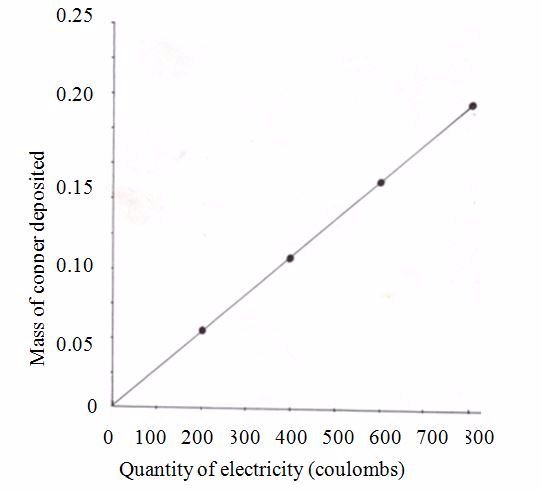
Graph of mass deposited versus amount of electricity passed
- m be the mass of the substance liberated;
- I be the current passed in amperes; and
- t be the time in seconds.
An Experiment to Verify Faraday’s Second Law of Electrolysis
The third (3) factor mentioned previously as affecting the amount of substance liberated during electrolysis may also be investigated experimentally. Because our interest is the effect of the charge on the ions present in solution, we need to keep the quantity of electricity fixed whilst varying the types of the ions in solution. This may be achieved by passing the same quantity of electricity through two cells, with ions of different charges in each cell.
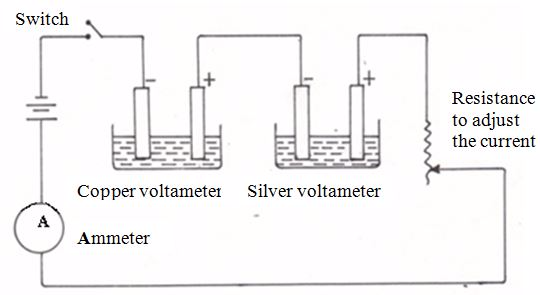
Apparatus for confirmation of Faraday’s Second Law
Specimen results
| Current flowing | = | 0.45A |
| Duration of current flow | = | 25 minutes |
| Mass of copper deposited | = | 0.221g |
| Mass of silver deposited | = | 0.755g |
- One faraday (IF) discharges one mole of H+, Na+, Ag+, Cl– and OH– ions.
- Two Faradays (2F) discharges one mole of Cu2+, Pb2+, Mg2+, Ca2+, Fe2+, etc ions.
- Three Faradays (3F) discharge one mole of Al3+, Fe3+, etc. ions.
Relationship between the Chemical Equivalents of Elements and Quantity of Electricity Passed
- mass of copper deposited
- mass of oxygen liberated
- Atomic weight of copper = 63.5
- Atomic weight of oxygen = 16
- 1 Faraday = 96500 C
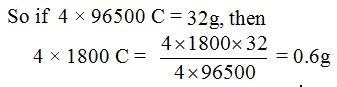
Application of Electrolysis
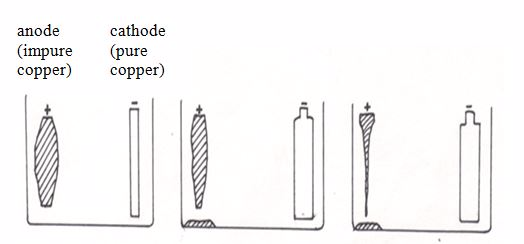
An Experiment on Electroplating of Metallic Materials
Electroplating is the coating of a metal with a layer of another metal by means of electrolysis. Electrolysis can be used to coat a thin layer of a less reactive metal onto a more reactive metal. The thin layer of less reactive metal will provide protection from corrosion for the more reactive metal underneath. It may also make the product more attractive.
Steel can be electroplated with chromium or tin. This prevents the steel from rusting and gives it a shiny, silver finish. This is also the idea behind chromium-plating articles such as car bumpers, kettles, bath taps, etc. Chromium does not corrode, it is a hard metal that resists scratching and wear, and can also be polished to give an attractive finish.
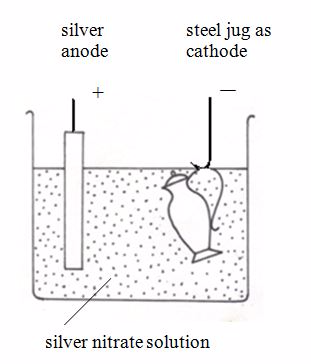
- Cathode – object to be electroplated
- Anode – metal M
- Electrolyte – solution of a soluble compound of M

































Leave a Reply