Topic 1- Oxygen – Chemistry Form Two
Oxygen exists in air to an extent of 21% by volume (or 23% by weight). It is the most abundant element on earth, accounting for ½ the total mass of the earth’s crust. Oxygen is mainly found in combined states as oxides, hydroxides, silicates, sulphates, carbonates, water, etc. Its ease of combination with other elements to form compounds shows that oxygen is a very reactive element.
Preparation and Properties of Oxygen
(i) Laboratory preparation of oxygen from hydrogen peroxide solution
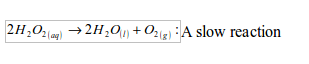
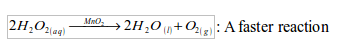
Preparation method


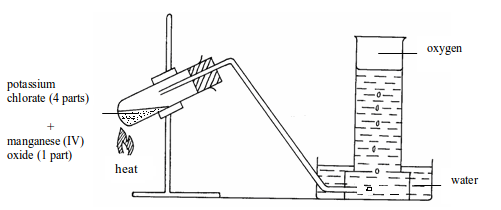
(ii) Laboratory preparation of oxygen from potassium chlorate

Preparation method
Test for oxygen
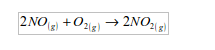
Simple Experiments to Demonstrate Properties of Oxygen Gas
1. Action of oxygen on metals

Reaction with specific metals
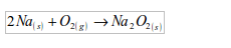
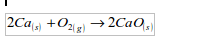
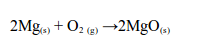
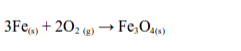
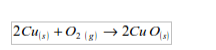
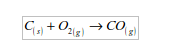
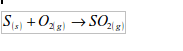
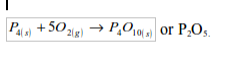
- It is a clear, colourless gas with no smell.
- It is a neutral gas (it is neither basic nor acidic in character)
- It is slightly soluble in water (100 cm3 of water at room temperature dissolves about 4 cm3 of oxygen).
- It has almost the same density as water although slightly denser than air. 5. It boils at -183ºC and freezes at –218ºC.
- Oxygen supports combustion
- It is a very strong oxidizing agent.
- Oxygen is very reactive. It reacts vigorously with a great many metals and non-metals to form basic and acidic oxides respectively. Metal + Oxygen gives metallic oxide (most of these are basic in character). Non-metals + Oxygen gives non–metallic oxide (most of these are acidic in character).
Uses of Oxygen
- The oxygen in the air and that dissolved in water and soil is used by all respiring organisms. Also all types of burning need oxygen.
- It is used in the oxyacetylene (oxygen–ethyne) flame for welding and cutting steel.
- It is extensively used for removing impurities from pig iron in order to produce steel. Oxygen is blown into molten iron to remove impurities such as carbon or phosphorus, which are expelled in the form of gases, i.e. their oxides.
- Oxygen is used as an aid to breathing in hospitals, high altitude climbing or flying, and in deep sea diving.
- Liquid oxygen is used in the burning of fuels such as kerosene, hydrogen and hydrazine used in various types of rockets.
- It is used in the L-D process for making steel.
Relate some uses of oxygen to its properties






























Leave a Reply