Topic 1- Non Metals and Their Compounds – Chemistry Form Four
General Chemical Properties of Non Metals
The following are important chemical properties of non-metals which are connected with their tendency towards electron gain in the course of formation of compounds:



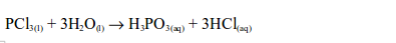

The Oxidizing Properties of Non-metals
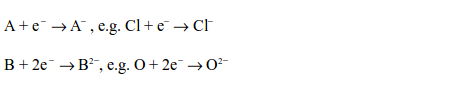
Strong and weak oxidants


The Displacement of Non-metals by another Non-metal from a Compound
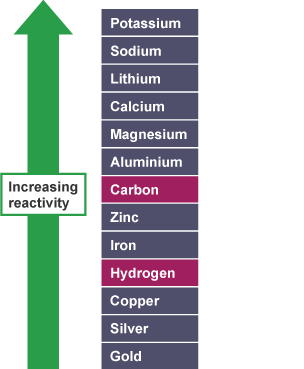
Chlorine
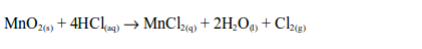
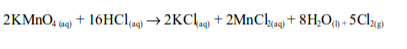
The poisonous nature of chlorine
Chlorine is a useful but dangerous gas. It is very poisonous if inhaled to even a small extent (1 part of chlorine in 50,000 parts of air causes death). Chlorine poisoning occurs when the gas is inhaled or swallowed. It reacts with water inside and outside of the body (such as water in the digestive tract and moisture on the lungs and eyes) to form hydrochloric acid and hydrochlorous acid. Both of these substances are extremely poisonous.
The Chemical Properties of Chlorine
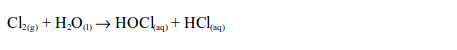
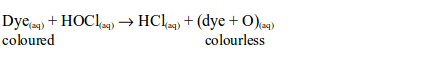
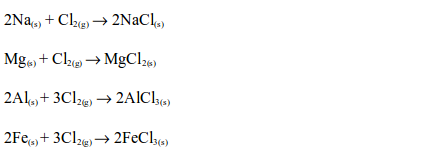

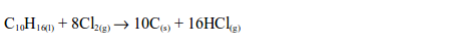


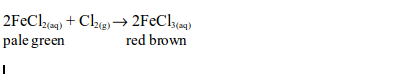
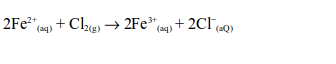
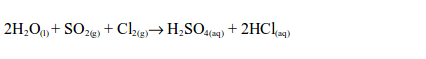

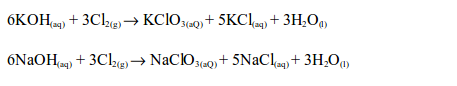
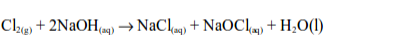
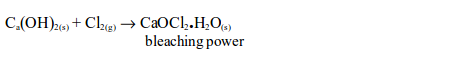
Hydrogen Chloride
Prepare a dry sample of hydrogen chloride gas
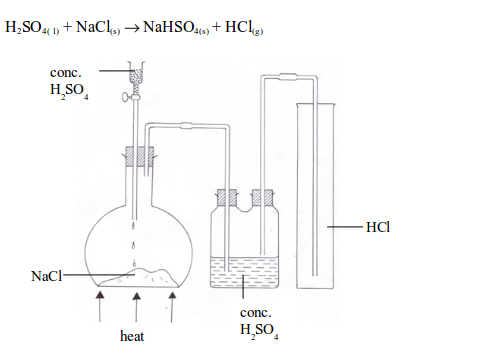
The Properties of Hydrogen Chloride Gas
- It is a colourless gas with a choking, irritating smell and an acid taste.
- It is heavier (denser) than air.
- It fumes in most air due to the formation of tiny droplets of hydrochloric acid.
- It is very soluble in water (450 cm3 of gas in 1 cm3 of water). The acidic properties and the solubility of hydrogen chloride gas can best be shown by the fountain experiment

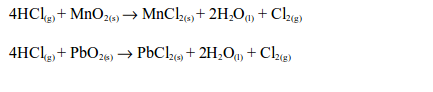


Reactions of aqueous hydrogen chloride

- it turns damp blue litmus paper red;
- it reacts with various substances just like other acids (see table bellow); and
- it conducts electricity, yielding hydrogen gas at the cathode and chlorine gas at the anode.
Reactions of aqueous hydrogen chloride
| Acid reacting with | General equation |
| oxide (base) | acid + metal oxide ® salt + water |
| alkali (soluble base) | acid+metal hydroxide(akali)®salt+water |
| metal | acid + metal ® salt + hydrogen |
| metal carbonate | acid + metal carbonate®salt+water+CO2 |
The Uses of Hydrogen Chloride
- It is chiefly used in the production of hydrochloric acid. When the gas is dissolved in water in the appropriate proportions, hydrochloric acid is formed.
- Aqueous hydrogen chloride is used in qualitative and quantitative analysis.
- It is an important reagent in other industrial chemical transformations, e.g. hydrochlorination of rubber and production of vinyl and alkyl chlorides.
- In the electronics industry, it is used to both rub semiconductor crystals and to purify silicon.
- It is used in the textile industry, to separate cotton from wool and fluff.
- In the laboratory, anhydrous forms of the gas are particularly useful for generating chloride-based Lewis acids.
- It is used to remove rust from the oxidized metals.
- It is extensively used in the manufacture of medicines and is a key substance utilized to turn maize and other agricultural products into artificial sweeteners.
Sulphur
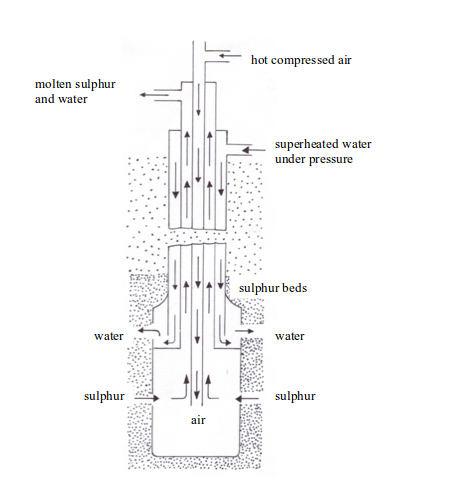
- Superheated water (170oC) is pumped through the outer pipe to melt the sulphur.
- Hot compressed air (10 atm) is pumped down through the inner pipe. The combination of the hot water and the hot air melts the sulphur. The molten sulphur, hot air and hot water form a froth.
- The froth is forced to the earth’s surface through the middle pipe by the compressed air.
- The molten sulphur is collected in large tanks (where the water drains off), cooled and solidified.
The Properties of Sulphur
1. Oxidizing properties of sulphur




2. Reducing properties of sulphur
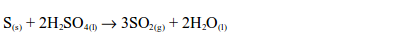

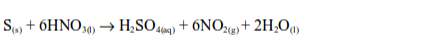
The Uses of Sulphur
Includes
- Most of the sulphur produced in the world (90%) is used to manufacture sulphuric acid. Sulphuric acid is an important reagent in many industrial processes.
- Sulphur is used in the manufacture of sulphur dioxide (used in the Contact Process for the manufacture of sulphuric acid).
- Manufacture of calcium hydrogensulphite, Ca(HSO3)2, and sodium sulphite which are used for bleaching wood straw and wood pulp in the paper industry.
- It is also used for vulcanization of natural rubber. Rubber is usually sticky and soft. When heated with sulphur (vulcanization), it becomes hard and strong.
- It is used for dusting vines to prevent growth of certain kinds of fungi and also as an insecticide.
- Sulphur is used in smaller quantities for the manufacture of dyes, explosives, fireworks, gunpowder etc. For example gunpowder is a mixture of potassium nitrate, carbon and sulphur.
- It is used in the manufacture of various organic compounds such as plastics and pharmaceuticals like sulphur ointments e.g. sulphadimadine, septrin e.tc.
- Photographic chemicals such as carbon disulphide (CS2) and sodium thiosulphate (Na2S2O3) are made using sulphur as one of the raw materials.
- Some is added to cement to make sulphur concrete. Unlike ordinary cement, this is not attacked by the acid. So it is used for walls and floors in plants where acid is used.
Sulphur Dioxide
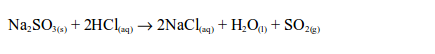
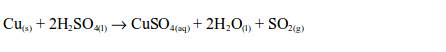
- The gas is colourless with an irritating (pungent), chocking smell.
- It is denser than air. Its density is 2½ times that of air.
- It is readily soluble in water and forms an acidic solution of sulphurous acid.
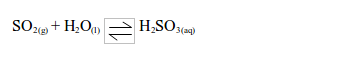
Acidic characteristics of sulphur dioxide
Chemical properties
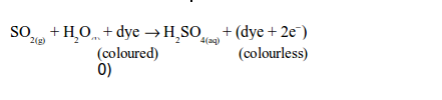

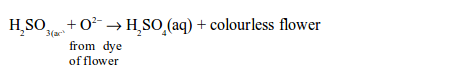
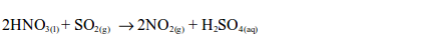
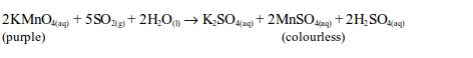
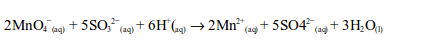
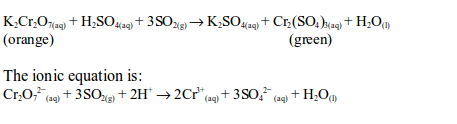
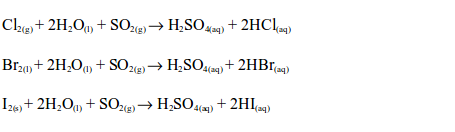
The oxidizing properties of sulphur dioxide
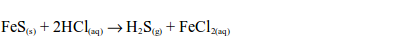
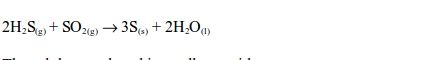

- The gas can be identified by its characteristic pungent and choking smell.
- It can also be detected by putting into it a filter paper that has been previously dipped into an acidified solution of potassium dichromate (VI). The colour of the filter paper changes from orange to green due to the reduction of dichromate (VI) to chromate (III).
- Sulphur dioxide also decolourized acidified potassium permanganate solution.
- The gas is used in the industrial manufacture of sulphuric acid in the Contact Process.
- It is used as a bleaching agent for wood pulp, silk, wool and straw.
- Its poisonous nature makes it a useful fumigant. So it is used in fumigation. The gas is poisonous to all organisms, particularly bacteria.
- It is used as a preservative and sterilizing agent in making soft drinks and jam, and in dried fruits. A very low concentration of the gas in food prevents fermentation as it stops the growth of bacteria and moulds. Its reaction with oxygen prevents oxidation of juices and other liquids to which it is added for preservation.
Hazards of sulphur dioxide gas

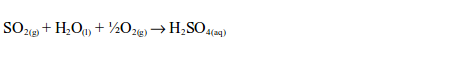
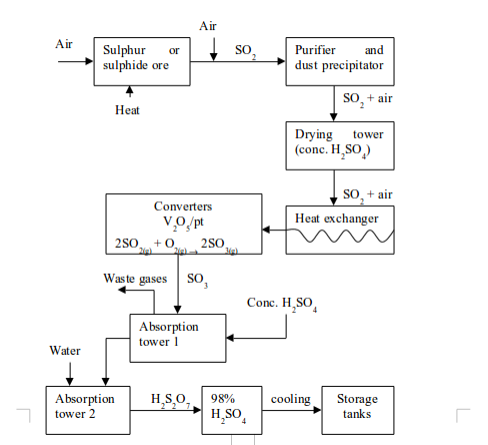
Sulphuric Acid
- production of sulphur dioxide;
- purification of sulphur dioxide;
- catalytic conversion of sulphur dioxide (SO2) to sulphur trioxide (SO3); and
- conversion of sulphur trioxide to sulphuric acid.


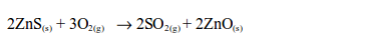
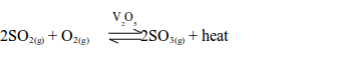


Reaction with metals
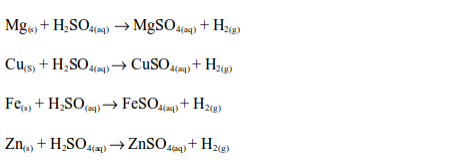
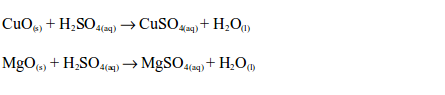





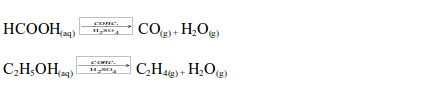
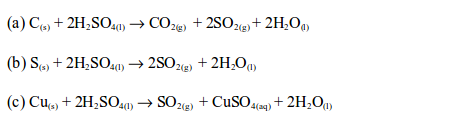

The Uses of Sulphuric Acid
Manufacture of fertilizers The major use of sulphuric acid is the production of fertilizers such as ammonium sulphate and superphosphates (phosphate fertilizers).
Manufacture of chemicals It is widely used in the manufacture of chemicals e.g. in making hydrochloric acid, nitric acid, phosphoric acid, sulphate salts, synthetic detergents, soap, paints and pigments, explosives, plastics and drugs.
Refining of crude oil A large quantity of sulphuric acid is used in refining crude oil.
Extraction and manufacturing of metals Sulphuric acid is used in the iron and steel-making industry to remove rust and scale from the surface of the rolled iron sheets. It is also used in processing metals e.g. in pickling (cleaning) iron and steel before plating them with tin or zinc to produce galvanized iron.
Manufacture of alum Sulphuric acid is used in the manufacture of aluminium sulphate, which is used in water treatment plants to filter impurities and to improve the taste of water. Aluminium sulphate is made by reacting bauxite with sulphuric acid.
Manufacture of natural and man-made fibres Sulphuric acid is used for making natural and synthetic (artificial) fibres. For example, the textile called rayon is made from cellulose fibres derived from wood. These fibres are dissolved in a solution of tetraamminecopper (II) sulphate to produce a thick liquid which is then injected into sulphuric acid to form rayon fibres.
Other uses:Sulphuric acid is used as (i) an electrolyte in lead-acid batteries, which are used in cars, to allow the flow of electrons between the plates in the battery. The sulphuric acid used in this way is called battery acid; (ii) as a general dehydrating agent in its concentrated form in tanning leather; and (iii) in waste water treatment.
Nitrogen


Tests for nitrogen gas
- Remove the cover from the first jar and smell the gas. Observe the colour of the gas and identify its smell.
- Remove the cover from the second jar and put in it a piece of damp universal indicator.
- Place a lighted splint into the third gas jar.
- To the fourth jar, add some calcium hydroxide solution (lime water) and shake.
- The gas is colourless and odourless. This distinguishes it from gases such as sulphur dioxide, ammonia, hydrogen chloride, etc.
- The colour of the indicator does not change. This shows that nitrogen is a neutral gas.
- The lighted splint is extinguished and the gas does not burn. It can not, therefore, be any gas which supports combustion, e.g. oxygen, dinitrogen oxide, or any combustible gas, e.g. hydrogen sulphide, carbon monoxide, hydrogen, etc.
- After the above tests, the only gas with which nitrogen may be confused is carbon dioxide. To distinguish it from carbon dioxide, the gas is dissolved in calcium hydroxide solution. Nitrogen leaves the calcium hydroxide unchanged while carbon dioxide turns the solution milky (due to formation of a precipitate of CaCO3).
Properties of nitrogen



The Uses of Nitrogen
Manufacture of fertilizers: Nitrogen is used to manufacture nitrogenous fertilizers. These include diammonium phosphate (DAP), calcium ammonium nitrate (CAN), ammonium superphosphate (ASP), ammonium nitrate (AN), ammonium phosphate sulphate (APS), ammonium sulphate nitrate (ASN), ammonium sulphate (AS) and urea.
Refrigeration: Because of its low boiling point (-196oC), liquid nitrogen is used as a refrigerant for storing organs in research laboratories, bull semen for artificial insemination, eggs and other cells for medical research and reproductive technology, etc. It is also used for preservation of food products and for their transportation.
Processing reactive substances: Some reactions require an inert atmosphere in order to proceed as desired. Because of its low reactivity, nitrogen is used to provide an inert atmosphere for storing and processing reactive substances.
Manufacture of nylon: Nitrogen is used in the manufacture of synthetic fibres such as polyamides. Polyamides are commonly known as nylons. Nylons are chemically inert and are stronger than natural fibres. They are used in making fishing nets, clothes, ropes, and many other items.
Manufacture of ammonia: Nitrogen is used in the manufacture of ammonia through the Haber Process.In the Haber Process, ammonia is manufactured by direct combination of nitrogen and hydrogen.Nitrogen and hydrogen are mixed in the ratio of 1:3. The gases are then reacted together at a temperature of about 450°C and a pressure of 250 atmospheres in the presence of finely divided iron as a catalyst. N2(g) + 3H2(g) ⇔2NH3(g) + heat The gases are cooled while still under pressure and ammonia is removed as a liquid.
Manufacture of nitric acid: The ammonia gas manufactured using nitrogen in the Haber Process is used in the manufacture of nitric acid by catalytic oxidation.
Plant nutrition: When atmospheric nitrogen is fixed into the soil by bacterial actions, it becomes a nutrient to plants. Nitrogen fixation refers to the conversion of atmospheric nitrogen, by certain species of bacteria, into nitrites.
Ammonia
Laboratory preparation of ammonia
The Properties of Ammonia
Describe the properties of ammonia
- It is a colourless poisonous gas with a strong chocking smell.
- It is less dense than air.
- It is easily liquefied by cooling to -33°C or by compressing it.
- It turns wet, red litmus paper blue as it is the only common alkaline gas
- It is very soluble in water forming alkaline solution. Ammonia has the highest solubility of all known gases. About 800 volumes of the gas dissolve in 1 volume of water at 15°C. The fountain experiment below demonstrates this solubility.
- Burning ammonia in an oxygen-rich atmosphere; and
- Use of a catalyst.
Using a catalyst
Reaction with copper
Reaction with hydrogen chloride gas
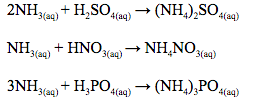
- Ammonia comes as a gas or a concentrated solution which is less easy to store; it is easier to store the solid ammonium salts.
- Ammonia is alkaline and can affect the natural pH of the soil.
- Ammonia easily evaporates if directly applied to the soil.
Manufacture of nitric acid
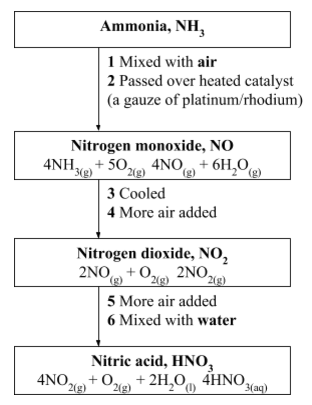
Cleaning
Refrigeration
- All living things are made of carbon and its compounds.
- Over ¾ of the world’s power is obtained from carbon and its compounds.
- Over ¾ of all substances in the world are made of carbon.
- graphite;
- diamond; and
- amorphous carbon.
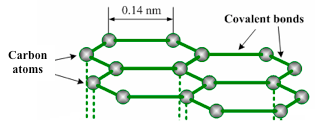
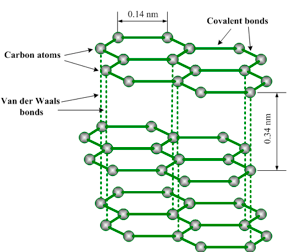
Graphite structure
- It is a black, soft and slippery substance. It feels soapy and greasy. It has a metallic lustre and is opaque to light.
- It has low relative density (2.3) as compared to diamond (3.5)
- Graphite is a good conductor of heat and electricity due to the delocalized electrons.
- It has a very high melting point (3730°C) and boiling point (4830°C). The melting and boiling points are high because of strong covalent bonds between the carbon atoms which require more energy (heat) to break in order to melt graphite.
- It is used as an electrode in electrolysis and as a positive terminal in dry cells. The use of graphite as electrode in electrolysis has an advantage because it does not react readily with most substances (it is an inert electrode).
- It is used as a lubricant, particularly when high temperatures are involved, where the usual lubricating oils easily decompose due to extreme heat. It is a lubricant for dynamos, electric motors and fast-moving parts of machinery.
- The major use of graphite is in making lead pencils of different hardness, by mixing it with different proportions of clay. The weakly held layers of carbon atoms in graphite easily slide over each other and are left behind on paper as black marks.
- Being resistant to chemicals and having a high melting point and also because it is a good conductor of heat, graphite is used to make crucibles.
- Graphite has the ability to absorb fast-moving neutrons, thus, it is used in nuclear reactors to control the speed of the nuclear fission reaction.
Diamond
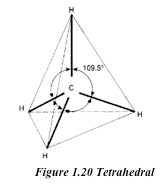
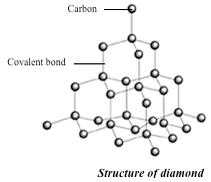
- It is the hardest natural substance known. This due to the strong covalent bonds between the carbon atoms in diamond. Again the compact tetrahedral arrangement of carbon atoms contributes to its hardness.
- It has the highest melting point (3550°C) and boiling point (4289°C).
- It has a high relative density (3.5) compared to graphite (2.3)
- It is a poor conductor of heat and electricity. This is because there are no free electrons to conduct electricity. All electrons are firmly held in covalent bonds.
- It is colourless, transparent and has a dazzling (amazing) brilliant lustre and radiance.
- It has a high refractive index of 2.5. The high refractive index results in high dispersion of light, making it suitable for use in jewellery.
- It is used in making jewellery.
- Due to its extreme hardness, it is used to make glass cutters, drilling devices, rock borers, and as an abrasive for smoothing very hard materials.
Comparison of the properties of diamond and graphite
| Diamond | Graphite |
| 1. Colourless, transparent andglittering | Black, opaque with metallic lustre |
| 2. Hardest natural substanceknown, used to cut glass andin drills | Soft to touch, greasy or soapy, can be used as a lubricant and in making lead pencils |
| 3. High relative density (3.5) | Low relative density (2.3) |
| 4. Non-conductor | Good conductor of heat and electricity |
| 5. Burns in air least readily (atabout 900°C) | Burns in air readily (at 700°C) |
| 6. Have strong C-C covalentbonds arranged octahedrally toform a giant molecular crystal | Have strong C-C bonds within the hexagonal rings in the sheets but weak Van der Waal’s forces in between layers. |
| 7. Its cleavage is difficult and itoccurs along the boundaries ofthe octahedral crystal unit | Cleavage easy, and occurs along the sheets or the layers. |
| 8. Prepared from graphite at very high pressure and temperature | Prepared from coke and silica mixture at high temperature |
| 9. Not attacked by potassiumchlorate and nitric acid together | Attacked by these reagents |
- Charcoal
- Lampblack (soot) or carbon black
- Coke
- Because of its ability to absorb large amounts of gas or liquid, it is used in gas masks to absorb poisonous gases in air in industrial process to recover volatile materials from waste gases.
- Wood charcoal can be used in metal refining instead of coke.
- Wood charcoal is a good source of energy. Thus, it is used as fuel for cooking and heating in homes.
- It is used in making printers’ ink, shoe polish and carbon papers.
- It is an important industrial material in the manufacture of tyres. It is used as a filler material in tyres.
Coke
- Coke is used as a fuel and as a reducing agent in the extraction of iron, lead and zinc. It is also used as a fuel in boilers.
- It is used in the manufacture of producer gas and water gas.
- Carbon burns in excess oxygen to form carbon dioxide C(s) + O2(g) → CO2(g).In insufficient oxygen, carbon monoxide is formed.2C(s) + O2(g) → 2CO(g)
- Carbon has got reducing properties and thus acts as a reducing agent. Carbon reduces oxides of metals below it in the electrochemical and activity series to their respective metals. This occurs on strong heating, and this reaction is used industrially for extraction of metals from their ores:PbO(s) + C(s) → Pb(s) + CO(g);Fe2O3(s) + 3C(s) → 2Fe(s) + 3CO(g);ZnO(s) + C(s) → Zn(s) + CO(g).
- Sulphur vapours react with red hot carbon to give carbon disulphide. C(s) + 2S(g) → CS2(l)
- Carbon dioxide is reduced by red hot carbon to carbon monoxide C(s) + CO2(g) → 2CO(g);This reaction is used in the industrial manufacture of producer gas.
Carbon Dioxide
Prepare a dry sample of carbon dioxide gas in the laboratory
Laboratory preparation of carbon dioxide
The Properties of Carbon Dioxide
- Carbon dioxide is a colourless and odourless gas.
- It is denser than air.
- When the gas is cooled to –78°C, it turns straight into the solid (it sublimes). Sublimation is the change of a gas straight into a solid or change of a solid straight into a gas. Solid carbon dioxide is called dry ice. It sublimes when it is heated or exposed to air.
- It has a melting point of –199°C and boiling point of –91.5°C.
- Carbon dioxide does not support combustion. This is why it is used in fire extinguishers.
Chemical properties
Reaction of carbon dioxide with water
The Uses of Carbon Dioxide
Uses of Carbon Dioxide include:
Fire extinguisher: Carbon dioxide is inert (i.e. it does not burn). It is dense than air and does not support combustion. Hence, it is a very useful fire-fighting chemical. When applied to fire, it forms a blanket over the burning material. Thus, it prevents air (oxygen) from reaching the burning material and therefore, extinguishing the flames.
Manufacture of aerated (fizzy) drinks: Soda water and mineral water contain carbon dioxide dissolved under pressure. Because the gas is only slightly soluble, it is bubbled into these drinks under pressure to make more of it dissolve. When the bottles are opened, the gas escapes and it causes the “fizzy”.Dissolved carbon dioxide is responsible for the pleasant taste of soft drinks such as lemonade, Coca cola, Pepsi cola and other aerated drinks and mineral waters. Other beverages like beers are also bottled together with carbon dioxide.
Refrigeration: Carbon dioxide is used for refrigeration purposes (i.e. in the deep-freezing of foods). The gas liquefies at ordinary pressure to form dry ice which sublimes at -78°C. Dry ice is a good refrigerant because it leaves no liquid after sublimation as is the case with ordinary ice.
Manufacture of sodium carbonate by the Solvary Process:Carbon dioxide is used in the manufacture of anhydrous sodium carbonate in the Solvary Process. The sodium carbonate produced is used in the manufacture of glass.
Manufacture of baking soda: Carbon dioxide is used in making baking soda (sodium bicarbonate). Baking soda is prepared by passing carbon dioxide into cold concentrated sodium hydroxide solution: CO2(g) + 2NaOH(aq) → Na2CO3(aq) + H2O(l).Further addition of carbon dioxide produces sodium bicarbonate which, at sufficiently high concentration, will precipitate out of the solution as a solid: Na2CO3(aq) + CO2(g) + H2O(l) → 2NaHCO3(s) Yeast and sodium bicarbonate (hydrogencarbonate) are important in the baking industry. Thus in baking of bread, yeast is added to flour, sugar and water (forming the dough). In the making of cakes, baking powder (a mixture of bicarbonate and an acid) is used instead of yeast.
Rain making: When pieces of dry ice (solid carbon dioxide) are dropped into clouds, the temperature of the clouds is lowered to such an extent that rain precipitates out.
Photosynthesis: Plants use carbon dioxide from the air to manufacture their own food through the process of photosynthesis.

































Leave a Reply