Topic 6: Mechanical Properties of Matter – Physics Form One Notes New Syllabus
Mechanical Properties of Matter
Study of mechanical properties of matter plays a significant role in physics and engineering. When constructing houses, bridges, and fly-overs, the proper selection of materials, based on their properties, is required.
In this lesson, the concepts involving elasticity, adhesion and cohesion, surface tension and capillarity will be discussed. The competencies developed will enable you to analyse and apply the properties of different materials found in your environment.
Concept of elasticity
If you stretch and release a rubber band, it will return to its original shape and size. If you compress and release a coil spring, it will resume its original length and shape. When a rubber band is stretched, we say it is deformed because it is not in its original shape.
A coil spring is also deformed when compressed. The ability of objects to return to its original shape after deformation is called elasticity. Thus, elasticity is the ability of a deformed body to return to its original shape and size when the forces causing the deformation are removed. A body with this ability is said to be elastic.
Relationship between tension and extension of an elastic material
When a metal is subjected to a force it undergoes deformation. The force that causes this deformation is referred to as tension, while the extent of deformation is known as extension.
Elastic materials undergo deformation when tension is applied but regain their original shape when the tension is released. Materials which cannot exhibit elasticity are known as inelastic materials. Examples of elastic materials include a rubber band and strings. A good example of inelastic material is the glass. Figure 6.1 shows some elastic materials.



To a great extent, most materials exhibit elastic behaviour. However, there is a limit of how much the material can deform and be able to regain its original shape. This limit is known as the elastic limit. Different materials have different elastic limits.
If a material is deformed beyond the elastic limit, it behaves as plastic. Such a deformation is known as plastic deformation. Therefore, if the elastic limit is not exceeded, there is a definite relationship between tension and extension of elastic materials. This relationship can be realized by performing Activity 1 below.
Activity 1
Aim: To investigate the relationship between tension applied to an elastic material and extension.
Materials: Coil spring, pointer, slotted masses, mass holder, metre rule, retort stand and clamp
Procedure
1. Hang a coil spring on a retort stand as shown in Figure below.

2. Attach the pointer at the free end of the spring and hang the mass holder.
3. Place a metre rule adjacent to the apparatus so that the position of the pointer can be measured.
4. Measure and record the position of the pointer when there is no mass placed on the holder as X0.
5. Place a 50 g mass as shown in Figure 6.3 on the holder, measure and record the pointer position as X1.
6. Repeat the experiment with masses of 100 g, 150 g and 200 g. Record the respective pointer positions as X2, X3 and X4.

7. Tabulate the results of your observation in Table below.

Gravitational acceleration (g) = 0.01 N/g
Questions
(a) Comment on the values of F/e
(b) What is the relationship between tension and extension?
(c) Plot a graph of force (F) against extension (e) of the spring.
(d) Give an explanation for the shape of the graph.
(e) Use the graph to determine the constant of proportionality in this case.

The graph of tension against extension is shown in Figure below.

Certain observations can be made from the graph in Figure 6.4. For the portion between O and A, the tension (force) is directly proportional to the extension of the spring. This is in accordance with Hooke’s law, which explains the relationship between the force applied on an elastic material and the extension produced.
Hooke’s law states that: “Provided the elastic limit is not exceeded, the extension is directly proportional to the force applied”.
The elastic limit is the maximum point beyond which the material cannot return to its original shape when the deformation force is removed. If the elastic limit is exceeded the material experiences plastic deformation.
From the graph, in Figure 6.4, point B is the elastic limit and the part between points B and C, the spring undergoes plastic deformation, whereby it remains deformed even after the load is removed. The spring will continue to increase in length until a certain maximum tension is reached. Beyond this tension, the spring will finally break.
In the elastic region, the ratio of the applied force (F) to the distance stretched (e) is called the elastic force constant, k. Hence,
k = F/e
The SI unit of k is N/m.
Exercise 1
1. A force of 40 N is applied to the spring with a spring constant of 100 N/m. What is the resulting displacement of the spring?
2. A rubber band has an original length of 20 cm and is stretched to a length of 25 cm. What is the percentage change in length of the rubber band?
3. A spring has a spring constant of 80 N/m. If the spring is compressed by 30 cm, what is the resulting force applied to the spring?
Applications of elasticity in daily life
Elasticity has a variety of applications at home, in transport, and industry.
At home, the applications of elasticity can be observed in:
(a) rubber gaskets that seal the refrigerator door;
(b) clothing;
(c) springs in furnitures;
(d) rubber bands that hold things together; and
(e) toys like balloons and balls.

In transport, we find application of elasticity in:
(a) hoses, belts and shock-absorbing springs for cars and trucks;
(b) rubber tyres; and
(c) support cables for bridges.

In industry, the application of elasticity is found in:
(a) steel beams used in construction;
(b) conveyor belts;
(c) measuring weight;
(d) insulation against vibration and sound; and
(e) mechanical control devices.

Task 1
Identify some elastic objects in your environment and explain how the concept of elasticity has made them useful in your daily life.
Adhesion and cohesion
If you pass through a grass field in the morning you will notice some drops of water stuck on grass leaves. Water drops are formed by the molecules that stick together because of cohesive forces.
On the other hand, drops of water stick to the grass leaves because of the adhesive forces between water molecules and grass molecules. Thus, cohesion and adhesion are the two important concepts in explaining molecular interactions.
Cohesion refers to the force of attraction between molecules of the same type whereas adhesion refers to the force of attraction between molecules of different kinds.
Both cohesion and adhesion play important roles in explaining various physical phenomena. For example, solids are known to have definite shapes because of the strong cohesive forces among their molecules which are in fixed positions and close together.
Water wets glass surfaces since adhesive forces between water and glass molecules are stronger than cohesive forces between water molecules. Conversely, mercury does not wet glass surfaces since cohesion between its molecules is stronger than adhesion between mercury and glass molecules.
Furthermore, cohesion and adhesion explain why liquids form meniscus when poured into glass tubes as shown in Figure below.

Mercury forms a convex meniscus because of the stronger cohesion between its molecules compared to the adhesion between glass and mercury molecules. Water forms concave meniscus because adhesive forces between water and glass molecules are stronger than cohesion between water molecules.
Activity 2
Aim: To observe the adhesion and cohesion.
Materials: Water, glycerine, glass slides, cylinders and droppers
Procedure
1. Pour about 50 ml of glycerine and water into separate cylinders and carefully observe the meniscus of each liquid.
2. Using a dropper, place a drop of glycerine on glass slide and note down your observation.
3. Use another dropper to place a drop of water on another glass slide and note your observation.
Questions
(a) Compare the menisci of water with glycerin.
(b) What did you observe when drops of glycerin and water were placed on separate glass slides?
(c) Explain your observations.

The meniscus of water curves upwards forming a concave shape while the meniscus of glycerine curves downwardsforming a convex shape. When each of these liquids is dropped on a glass slide, the water spreads further unlike glycerine.
This is because glycerine has a high cohesive force among its particles. A drop of glycerine on a glass slide remains almost spherical because the cohesive force is stronger than adhesive force.
Applications of adhesion and cohesion
The following are applications of adhesion and cohesion:
1. If we want to stick two different objects together, we use adhesive effects of the tape or glue.
2. Adhesion helps to remove harmful materials such as bacteria from drinking water.
3. Adhesive forces are the source of friction between surfaces.
4. Cohesion supports water transport in plants and animals.
5. Animals and plants also use cohesion of tissue to repair damage.
6. Cohesion is responsible for viscosity.
7. Ink sticks on paper because adhesive forces between ink and paper are greater than the cohesive forces in the molecules of ink. This explains why your writing remains on the paper.

Task 2
Think of your daily life, paying attention to situations where cohesion and adhesion are involved. Discuss how these concepts are crucial to your daily activities.
Surface tension
Liquid surfaces have a tendency of shrinking into the minimum surface area. This tendency leads to surface tension.
Surface tension is defined as the property of the surface of a liquid that allows it to resist an external force due to the cohesive nature of its molecules.
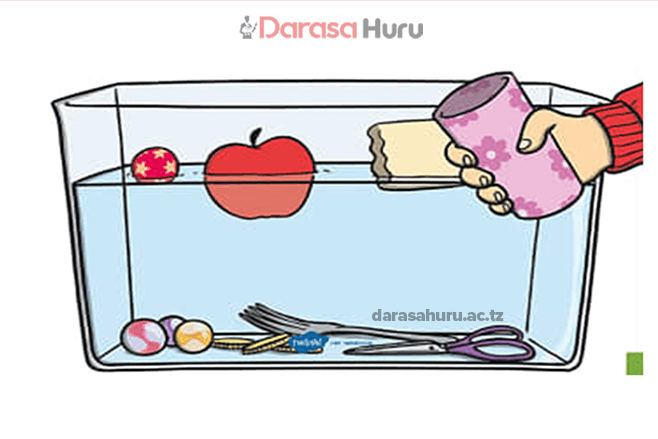
This allows the liquid surface to behave like a fully stretched elastic skin. Small insects such as water striders and items like plastic, paper clips float on the surface of water, shown in Figures 6.6 and 6.7, because of the surface tension of water.

Water strider

Paper clip floating on the surface of water
Mechanism of surface tension
Surface tension is caused by cohesive forces between liquid molecules. Taking an example of water in a glass, the molecules at the surface of water do not have neighbouring water molecules above them, as shown in Figure below.
Consequently, these molecules cohere more strongly to the water molecules directly associated with them, that is the molecules that are next to and below them. Because of the stronger cohesive force between water molecules, it is more difficult to move an object through the surface than to move it when it is completely submerged.
This resistance to an external force due to cohesive forces between molecules is called surface tension.

Activity 3
Aim: To demonstrate the surface tension in water.
Materials: Needle, water, trough, paper clip, chalk powder, liquid detergent and razor blades
Procedure
1. Fill the trough with water.
2. Gently place the needle, razor blade and paper clip on the water surface.
3. Observe what happens.
4. Sprinkle some chalk powder on the water surface.
5. Note down your observations.
6. Add a little detergent such as soap to the water in the trough and observe what happens.
Questions
(a) What did you observe when the needle, paper clip, razor blade and chalk powder were gently placed on the water surface?
(b) What did you observe when some detergent was sprinkled on the water?

When the needle, paper clip, and razor blade were placed on the surface of the water, they all floated on top, as shown using a razor blade in Figure 6.9. The surface tension of the water was large.

Razor blade floating in fresh water
However, when some detergent is added to the water, the same objects sink to the bottom of the trough, as shown using a razor blade in Figure 6.10. This means that the introduction of the detergent reduced the surface tension of the liquid.

Razor blade sinking in soapy water
If an object is placed on the surface of a liquid, its weight pushes the liquid downward causing a deformation, which increases the surface area of the liquid. The surface tension resists the increase in the area by pushing upward the object, as shown in Figure below.

Surface tension supporting an object
However, when some detergent is added in the water, the metal object sinks. This is because the detergent decreases the surface tension of the water. The water’s ability to push upward on the object is therefore lowered. The surface of the liquid expands and the object sinks to the bottom of the trough.
Detergents as examples of surfactants
A surfactant is a substance that reduces the surface tension of a liquid. The term ‘’surfactant’’ is an acronym for surface active agent.
Activity 4
Aim: To demonstrate surface tension in a soap film.
Materials: Wire, thin thread and soap
Procedure
1. Make a loop using the wire.
2. Make a smaller loop using the thin thread and suspend it in the wire loop, as shown in Figure below.

3. Dip the wire loop in a soap solution and let a film (skin) of soap cover the whole area of the loop.
4. Gently pierce the soap film using a hot pin inside the loop of the thread until it bursts.
Questions
(a) What is the shape of the thread when the loop is dipped in a soap solution?
(b) What happens when the soap film inside the loop of the thread is pierced?
(c) Explain your observations.

The thread hangs loosely even after the wire loop is dipped in a soap solution. When pierced, the soap film inside the loop of the thread disappears but the one outside the loop remains intact. The thread is pulled outwards and forms a circular loop.
Before piercing, the thread hangs loosely since there is a film of soap both within and outside the loop of the thread. The surface tension does not cause any tension in the thread. The sum of the forces on the thread is zero. However, on piercing the film inside the thread loop, the sum of the forces is no longer zero.
The surface of the soap wants to be at a minimum so the thread is pulled outwards causing it to form a ring, as shown in Figure below.

Wire ring with a loop of thread in a soap film
Surface tension is what causes drops of water or soap bubbles to assume a spherical shape. Recall that surfacetension attempts to form the minimum surface area and a spherical shape has the smallest surface area for a given volume.
The surface tension of any liquid is affected by the following factors:
1. Nature of the liquid: Different types of liquids have different surface tensions. For example, mercury has a higher surface tension than water.
2. Impurities: Impurities in a liquid lower its surface tension.
3. Temperature: The surface tension of a liquid decreases with an increase in temperature.
Applications of surface tension
Surface tension has several practical applications:
1. The cleaning action of soap (detergents in general) is due to its ability to lower the surface tension of water. For example, when washing clothes, soap decreases the adhesion forces between the particles of cloth and those of dirt. As a result, the dirt is easily removed since both water and soap particles penetrate the pores of the fabric.
2. Mosquitoes normally lay their eggs on water. The eggs hang on the water surface. When a small amount of oil is poured into the water, it reduces the surface tension. This breaks the elastic film and the eggs are drowned and killed.
3. Hot soup has a lower surface tension than cold soup. As a result, hot soup spreads over a large area of the tongue. Hence hot soup is tastier than cold soup.
4. Soap bubbles that consist of a layer of water between two layers of soap film can have a large surface area because the soap acts to decrease the surface tension of water. This is how surfactants are used to enhance the cleaning ability of water. Surfactants are also used to make emulsions of two liquids oil and water, which normally do not mix.
5. In the extraction of impurities during laboratory processes.

Task 3
Float two matchsticks on the surface of the water. Touch the water surface between the matchsticks using a hot needle. Record and discuss your observations.
Capillarity
Capillarity is another consequence of cohesive and adhesive forces.
It is defined as a phenomenon in which liquid rises or falls in a narrow space such as a thin tube or in the voids of a porous material without any assistance from external forces.
The effect of capillarity can be seen in drawing liquid between hairs of a painting brush, in a thin tube, in porous materials such as sponges and some non-porous materials such as sand.
Mechanism of capillarity
Capillarity occurs because of the intermolecular forces between the liquid and the surrounding solid surfaces. If the diametre of the opening is sufficiently small, the combination of surface tension and adhesive forces between the liquid and the container act to propel the liquid through the opening. Note that, surface tension is due to cohesive forces. That is, capillarity is caused by both adhesion and cohesion.
A common apparatus used to demonstrate capillarity is the capillary tube. The capillary tube is a narrow tube with a fixed length. When the lower end of the tube is placed in water, adhesion occurs between the liquid and the tube walls. This pulls the liquid column along the tube wall. The height of the liquid column increases until the weight of the liquid column is sufficient to overcome the cohesive and adhesive forces that propel the liquid.
Factors affecting capillarity
Capillarity is mainly affected by the following factors:
The diametre of the tube or opening
The height of the liquid column is inversely proportional to the diametre of the tube or opening. The weight of the liquid column is proportional to the square of the tube’s radius. It follows that a narrower tube will draw a longer liquid column than a wider tube. For example, the water in the glass capillary tube will rise to a height approximated by
0.3 /d
where d is the diametre (in centimetre) of the tube. Thus, if a glass tube has a diametre of 0.5 mm, water will rise to a height given as;
h = 0.3 /0.05 cm
= 6 cm
Tubes of different diameters will result to water columns of different heights, as shown in Figure below.

Capillary rise in tubes of different diameters
Nature of the Liquid
Capillarity also depends on the nature of the liquid since it is governed by cohesive and adhesive forces. For example, when the same capillary tube is dipped in water and mercury, the mercury level in the tube falls while the water rises, as shown in Figure below.

Capillary rise and fall
Activity 5
Aim: To observe capillarity in different liquids.
Materials: Beakers, 0.5 mm glass tubes, water, cooking oil, ethanol
Procedure
1. Fill three beakers about half full of water, ethanol, and cooking oil.
2. Immerse a glass tube of 0.5 mm diametre into each liquid; make sure that the base of the glass tube in each liquid is at the same level, as shown in Figure below.

3. Measure and record the height of each liquid above the surface of the liquid in the beaker.
Questions
(a) Which liquid rose to the highest height?
(b) Which liquid had the least rise in height?
(c) Explain what this suggests about the adhesive and cohesive forces in each liquid.

Ethanol rises to the highest height while water rises the least. This suggests that ethanol has the strongest adhesive force than water and cooking oil. Also, ethanol has the lowest density. This means that capillary action is greater in ethanol.
Applications of capillarity
1. Capillarity is essential to plants and animals. In plants, it facilitates the transportation of water and nutrients from the roots to the leaves. In animals, it assists in the circulation of blood.
2. Capillarity promotes the movement of groundwater. This is in addition to the gravitational movement of water in the soil.
3. It is the principle on which paper and fabric towels work to absorb water. For example, soaking up moisture from the body using a towel is due to capillary.
4. Clothes worn in hot climate conditions use capillary action to draw perspiration away from the body.
5. In an oil or kerosene lamp, capillarity draws fuel up into the wick where it can be burnt.

Chapter summary
1. Elasticity is the ability of a deformed material to regain its original size and shape after the deforming force is removed.
2. Hooke’s law states that ‘provided that the elastic limit is not exceeded, the extension of elastic material is directly proportional to the force applied’.
3. Surface tension is the ability of the surface of a liquid to behave like a fully stretched elastic skin. This elastic nature of the surface of a liquid is caused by the attraction of the liquid molecules at the surface.
4. Capillarity is the tendency of a liquid to rise and fall in narrow tubes or to be drawn into small openings. It occurs when the adhesive forces between the liquid and the walls of the tube are larger than the cohesive forces between the liquid molecules.
Revision exercise
Section A
1. Answer all questions by writing the letter of the most correct answer
(i) Where on the graph is the limit of proportionality for an elastic solid?

(a) between O and P (b) at P (c) between P and Q (d) at Q
(ii) When a force is applied to a given material, the material returns to its original shape after the force is removed. This behaviour is termed as:
(a) Plastic deformation
(b) Elastic deformation
(c) Irreversible deformation
(d) Viscous deformation
(iii) A glass capillary tube is placed in a beaker of water. The water level inside the tube is higher than the water level outside due to:
(a) Cohesion
(b) Adhesion
(c) Surface tension
(d) Elasticity
(iv) A weight of 2.0 N is hung from a spring. The extension produced is 6.0 cm. The 2.0 N weight is removed and an 8.0 N weight is hung from the spring. The spring does not pass its limit of proportionality. What is the new extension of the spring?
(a) 6.0 cm (b) 24 cm (c) 36 cm (d) 48 cm
(v) If the radius of a capillary tube is reduced while keeping all other factors constant, how will it affect the capillary rise height?
(a) The capillary rise will increase.
(b) The capillary rise will decrease.
(c) The capillary rise will remain the same.
(d) The capillary rise will become unpredictable.
(vi) The graph in Figure 6.18 shows how the extension of a spring changes with the masses suspended from it when the spring is on planet X and when the spring is on planet Y.

Which conclusion can be drawn from these graphs?
(a) It is not possible to compare the gravitational field strengths on planets X and Y.
(b) The gravitational field strength on planet X is equal to the gravitational field strength on planet Y.
(c) The gravitational field strength on planet X is one-third of the gravitational field strength on planet Y.
(d) The gravitational field strength on planet X is three times the gravitational field strength on planet Y.
Section B
2. (a) State Hooke’s law and express it in mathematical form.
(b) An object with a mass of 500 g was hung from the spring with a force constant of 20 N/m.
(i) How far in centimetres would the spring stretch?
(ii) What is the mass of an object that stretches the spring 35 cm long? (take g = 0.01 N/g).
3. (a) Explain why water wets glass while mercury does not.
(b) Explain how the knowledge of adhesion and cohesion is useful in daily life.
4. (a) Explain why mosquitoes can manage to walk on the surface of water.
(b) Explain why when washing clothes, we use a soap/detergent.
5. (a) Differentiate between capillarity and surface tension.
(b) Which phenomenon is taking place when kerosene rises up a wick?
6. (a) What will happen when a narrow glass tube is dipped into mercury?
(b) What is meant by the term meniscus? Name the kind of meniscus formed in a glass container:
(i) case of water
(ii) case of mercury
7. Describe how you would verify Hooke’s law of elasticity. Which precautions do you take in verifying the law?
8. Imagine you have two glass capillary tubes, one with a narrower diametre and the other wider. Dip both tubes in water and you will notice that the water level in the narrower tube rises higher than the wider one. What can you conclude from this observation?
9. A force of 9.6 N stretches a spring by 6 cm while a force of 14.4 N stretches it by 9 cm. What force would be required to stretch the spring by 15 cm?
10. A group of Form One students is conducting an experiment to investigate capillary action. They place a small glass tube into a container of water and observe the water level inside the tube. They then add a small amount of detergent to the water in the container and observe the water level inside the tube again. What effect, if any, will the detergent have on the water level inside the tube?
11. When both a rubber band and a metal spring are stretched using the same force, it will be noticed that the rubber band stretches much more than the spring. Why does this happen, and what does it tell us about the concept of elasticity in physics?
12. When a 150 g object is hung from the scale the pointer moves down a distance of 3 cm as shown in Figure

The object is then placed on a table and reattached to the spring balance as shown in Figure

The scale is then pulled to the right and at the moment the object begins to move, the pointer is found to have moved 1.2 cm to the left. What is the force between the object and the table?



































Leave a Reply