Topic 8- Extractions of Metals – Chemistry Form Three
Occurrence and Location of Metals in Tanzania
Locations of Important Metal Ores in Tanzania
Most metals are found naturally as compounds called minerals. Rocks are made up of crystals of metals. An ore is a rock that contains enough of a metal compounds for it to be worth extracting the metal. The most common ores contain oxides. An example is the ore haematite, which contains iron (III) oxide. Some contain other metal compounds. Malachite contains copper (II) carbonate.
Chemical Properties of Metals
- Na →Na+ + e– (univalent)
- Mg →Mg2+ + 2e– (divalent)
- Al →Al3+ + 3e– (trivalent)
- They react with oxygen to form oxides. For example, magnesium burns in air to form magnesium oxide. Metal oxides are bases, which mean they react with water to form an alkaline solution and with acids to form salts.
- Metals form positive ions when they ionize. Consider the ionization of sodium, magnesium and aluminium in the above equations in which case ionization resulted into Na+, Mg2+ , and Al3+ ions respectively. However, there are some exceptions. For example, hydrogen is a non-metal which forms positive ions in solution, H+. This is the only exception in this case
The reactivity and tensile strengths of some metals
- Calcium:Calcium is amongst a group of metals that are too reactive to occur in the free state. It occurs mainly as carbonate, sulphate, fluoride and silicate. It is a soft, greyish metal. In comparison with potassium and sodium, it has a lower tensile strength and high density.
- Iron:Iron is a typical metal. Its density is 7.87. It melts at 1530oC. Iron is a moderately reactive metal. The metal reacts with excess steam at red heat to produce triiron tetraoxide.
- Copper Copper is a less reactive metal. It is a red-brown metal with a lustre. It can be polished. Its tensile strength is fairly high. When heated in air, copper forms a layer of black copper (II) oxide on the surface: 2Cu(s) + O2(g)→2CuO(s)It reacts with hot concentrated sulphuric acid to form copper (II) sulphate and liberate sulphur dioxide. Cu(s) + 2H2SO4(aq)→CuSO4(aq) + 2H2O(l) + SO2(g)
Tensile strengths, densities and melting points of calcium, iron and copper
| Calcium | Copper | Iron | |
| Tensile strength | Low | Fairly high | High |
| Melting point (oC) | 850 | 1080 | 1535 |
| Density (g cm-3) | 1.55 | 8.95 | 7.9 |
The Reducing Power of Different Metals
As we learned early, metals tend to lose electrons during chemical reactions. This process of losing electrons is called oxidation. Metals normally lose electrons to non-metals, which accept those electrons. Therefore, metals are said to be electron donors while non-metals are electron acceptors. In this case, metals can be termed as reducing agents, because they donate electrons which, when accepted by non-metals, tend to lower their oxidation numbers. Non-metals are called oxidizing agents, because they oxidize or increase the oxidation number of metallic atoms through accepting the electrons donated by metals.
Reactivity series of metals
Reactivity series refers to arranging or listing the metals in order of reactivity. The reactivity series are obtained by consideration of the action of air, water and acids on the metals, and how easily the oxides of these metals can be reduced. Consider the table of reactivity series below (Table 8.2). Oxides of the first group of five metals cannot be reduced by carbon. Those of the second group of three metals can react with acids, displacing hydrogen.
The third and last group comprises of least reactive metals. In table 8.2, the metals are arranged in order of reactivity series. It indicates the inverse order in which the elements were isolated. Thus, metals low in the series such as gold, silver and lead have been known since early times. Metals high in the series proved very difficult to isolate. It was Davy’s work on electrolysis that led to isolation of potassium, sodium, calcium, magnesium and aluminium over a period of years from 1807, when Davy isolated potassium and sodium, to about 1850, when aluminium was isolated.
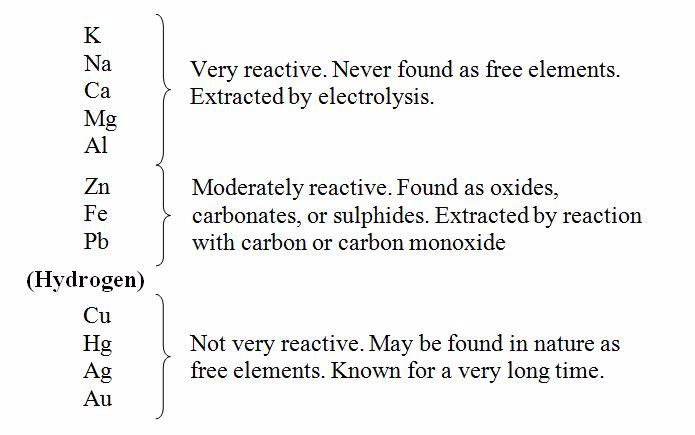
The reactivity series of metals
Useful things to remember about the reactivity series
- The more reactive the metal, the more compounds it forms. So only copper, silver and gold are ever found as free elements in the earth’s crust. The other metals are always found as compounds.
- When a metal reacts, it gives up electrons to form ions. The more reactive the metal, the more easily it gives up electrons.
- The more reactive the metal, the more stable its compounds are. Stable means difficult to breakdown. For example, when you heat sodium nitrate you get sodium nitrite:2NaNO3(s)→ 2NaNO2(s) + O2(g)But copper (II) nitrate breaks down further, to oxide, giving off nitrogen dioxide:2Cu(NO3)2(s)→ 2CuO(s) + 4NO2(g) + O2(g)
- The more reactive the metal, the more difficult it is to extract from its compounds (since the compounds are stable). For the most reactive metals, you will need the toughest method of extraction: electrolysis.
- The less reactive metals have been known and used since ancient times, because they are easiest to extract.6. If you stand two metals in an electrolyte and join them up with a copper wire, electrons will flow from the more reactive metal to the less reactive one.
Extraction of Metals by Electrolytic Reduction
For less reactive metals at the middle of the reactivity series, the oxygen can be removed by chemical reduction with carbon or carbon monoxide. This method is used for extraction of metals such as zinc, iron and copper as shown and discussed in table 8.3. Least reactive metals such as copper, silver and gold may be found in uncombined state.
Methods of extraction of different metals from their ores
| Metal | Method of extraction from ore |
| Potassium | Electrolysis |
| Sodium | |
| Calcium | |
| Magnesium | |
| Aluminium | |
| Zinc | Chemical reduction with carbon or carbon monoxide |
| Iron | |
| Lead | |
| Copper | Roasting in air |
| Silver | Occur naturally as elements |
| Gold |
Stages of the extraction of moderately reactive metals
- Mining and concentration of the ore
- Roasting in air
- Reduction of oxides to metals
- Purifying the metal
Mining and concentration of the ore
- It is crushed and washed. In this case, the ore is broken down into small pieces, which are then grinded down to fine powder. Then it is either dropped into water, where the fragments containing the metal sink faster or jets of air are blown at it, where the lighter waste material is carried to one side.
- A method called froth flotation is used with sulphide ores (e.g. CuS or ZnS). The ore is powdered, fed into water tanks and made into slurry with water. Then “frothing” chemicals (a suitable oil) are added. Sulphides are attracted to these chemicals. When air is blown through the slurry, froth rises to the top of the tank carrying the metal sulphides with it. They are skimmed off and dried. The gangue sinks.
- Magnetic separation can be used. The iron ore can be separated from other material in the crushed ore by using electromagnet.
Roasting in air
- 2PbS(s) + 3O2(g)→ 2PbO(s) + 2SO2(g)
- 2ZnS(s) + 3O2(g)→ 2ZnO(s) + 2SO2(g)
- ZnCO3(s)→ ZnO(s) + CO2(g)
Reduction of oxides to metals
Purification (Refining)
- Electrolysis: Electrolysis is used to produce a pure metal directly from its molten compounds. Examples of metals which are purified by electrolysis are copper and zinc. Copper produced in large scale is purified by electrolysis, a process often called copper refining.
- Oxidation: The molten crude metal is exposed to hot air in a furnace. The impurities in the crude metal are oxidized with oxygen from the air. They escape as vapour or form a scum over the molten metal, which is then removed by skimming. However, this method is used only when the impurities have a greater affinity for oxygen than the metal. The method is applied in the manufacture of steel from pig iron and in the purification of tin and lead.
- Distillation:In distillation, the crude metal is heated in a furnace until the pure metal evaporates, leaving behind the impurities. The vapour is then collected and condensed in a separate chamber. This method forms an integral part in the extraction of zinc, cadmium and mercury. A further distillation, usually in vacuum, gives a very pure product.
- Formation of carbonyls: very pure nickel and iron are made by forming their volatile carbonyls, which are then decomposed by heating.
- Zone refining: This recently developed method is used to produce silicon and germanium of extreme purity. In this method, a small high-frequency induction furnace is placed round one end of a long rod of the metal and a thin cross-section of the metal is melted. The furnace is then moved slowly along the rod. Pure crystals of the metal separate from the melting metal but impurities remain in the liquid and are carried along to the other end.
The Extraction of Sudium from its Ore
Explain the extraction of sodium from its ore
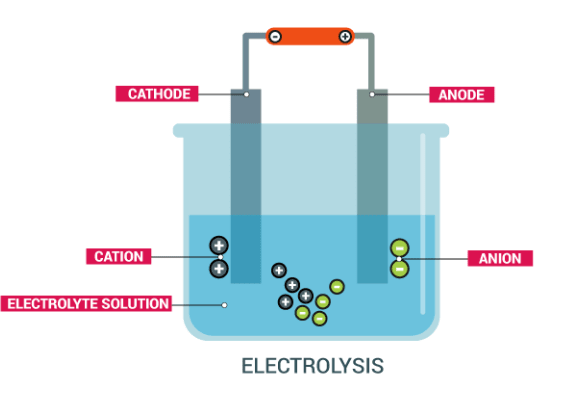
Alkali metals are strong reducing agents and cannot be extracted by chemical reduction of their oxides or other compounds. The only possible method of their extraction is by electrolysis of their fused chlorides.Sodium is extracted industrially by electrolysis of either fused sodium hydroxide (Castner’s process) or fused sodium chloride (Down’s process), in which sodium chloride is electrolysed in the molten condition.
Electrolysis of sodium chloride by the down’s process
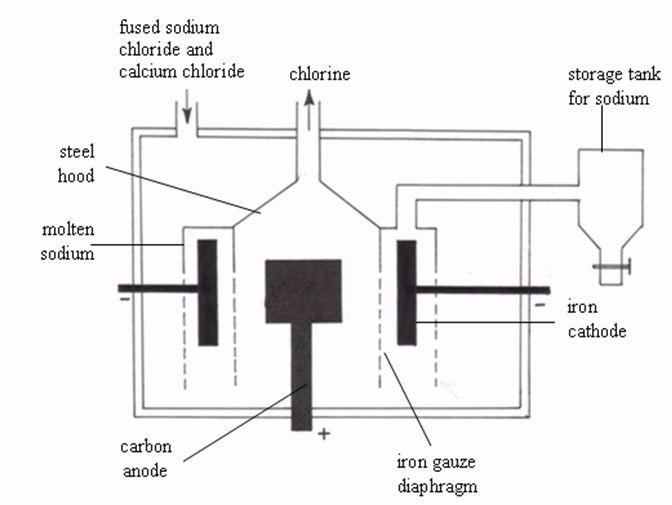
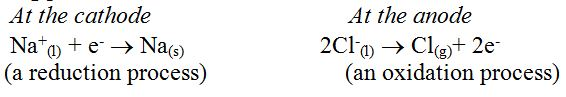
Extraction of Metals by Chemical Reduction
- Iron ore: The chief ore is haematite. It is mainly iron (III) oxide, Fe2O3 mixed with sand.
- Limestone: This is mainly calcium carbonate, CaCO3.
- Coke: This is made from coal and is almost pure carbon.
The extraction of iron in a blast furnace
Reactions that occur in the furnace
- At the bottom of the furnace where temperature is the highest, air attacks the coke to produce carbon dioxide.C(s) + O2(g)→ CO2(g)
- In the middle of the furnace, the rising up carbon dioxide gas is reduced by more coke, producing carbon monoxide.C(s) + CO2(g)→ 2CO(g)
- At the top of the furnace, carbon monoxide reduces iron (III) oxide to metal. Fe2O3(s) + 3CO(g)→2Fe(s) + 3CO2(g)
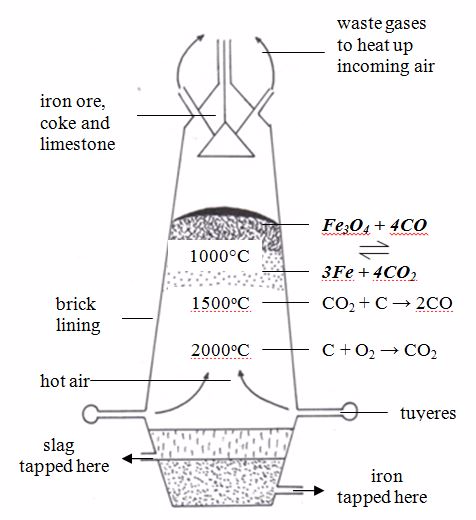
Environmental Consideration
The following are some of the environmental problems caused by mining:
Land subsidence (sagging): Holes created due to underground mining cause land to sink (or subside). This is because the holes underneath the ground cause imbalance in weight of the soil above the ground. This may result to severe damage to buildings and other infrastructures such as roads, railway trucks and so forth.
Poisonous substances: Poisonous compounds (for example of lead, cadmium and arsenic) are found in many ores. These may be washed into the soil and streams because of the mining process. If they happen to reach the water, they can kill fish and plant life, and can end up in your food as well. Gold extraction process makes use of mercury. If untreated effluent from the gold mine is directed to nearby rivers or streams, the metals may end up in fish, which might be someone’s food. Consumption of such fish can result to brain damage due to mercury contained in it.
Large volume of waste: Large-scale mining operations inevitably produce a great deal of waste. This waste not only comprises of earth from the soil and gangue but also includes the toxic chemicals added to the ore to aid metal extraction. The waste material gets washed into streams and rivers. The sediment that builds up blocks rivers and alters their routes. This serves as a source of pollutants to natural water systems.
Noise and dust: Mining activities produce a lot of noise and dust. Noise and dust can be caused by haulage trucks, rock blasting and crushing, drilling operations and heavy traffic. Everything for miles around the mine may get covered with dust.
Big holes in the ground: Mineral extraction leads to boring of deep holes through the ground in the course of searching for rich ores. Huge amounts of rock are dug up to get a small amount of ore. For example, 1000 tonnes of rock may produce just 5 tonnes of copper. This leaves huge scars on the landscape (if it is opencast method) or huge holes underground (if it is underground mining).
Great heaps of earth material: unwanted rock material, after the metal has been extracted from the ore, gets heaped up in tips. These are unsightly. They can be unstable and therefore dangerous. During heavy rains, a landslide is likely to occur, a catastrophe that often results to loss of life and destruction of property.
Soil erosion: Before mining operation is carried out, the natural vegetation on or around the mining site is usually cleared up in order to give enough room to mining activities. The consequent removal of vegetation cover leaves the soil bare and, therefore, susceptible to erosion.
Air pollution: Large-scale mineral extraction results to production of gases such as sulphur dioxide, carbon dioxide and other bad gases which are emitted to the atmosphere. These gases may bring about a green house effects and even cause acid rains.
Intervention Measures to Rectify Environmental Destruction
- Governments are getting ever tougher with mining companies about damage to the environment. Sadly, in developing countries like Tanzania where much mining takes place, laws may be less strict.
- Stern controls apply to the production of wastes that may be toxic or may cause environmental damage. Safety regulations and practices must be maintained to avoid the risk of accidental release of harmful materials.
- Mine reclamation activities are undertaken gradually with the levelling of the heaps of earth material, replacement of the top soil with a fertile one and planting of trees in the mined out areas. Care must be taken to relocate streams, wildlife and other valuable resources. Quarries and opencast workings can be reclaimed by the process of filling the holes with solid wastes. The eroded bare soil can be conserved by planting trees and grasses to serve as a soil cover, which would counteract the impacts of wind, running water, rain and animals to the soil.Reclaimed land can have many uses such as agriculture, forestry, wildlife, habitation and recreation.
- Dust levels can be controlled by spraying water on roads, stockpiles and conveyors. Other steps can also be taken including filling of drills with dust collection systems, and purchasing additional land surrounding the mine to act as a buffer zone. Trees planted in these buffer zones can also minimize the visual impact of dust, from the mining operations, to local communities.
- Noise can be controlled though careful selection of equipment and insulation, and enclosures around machinery.
- The poisonous and toxic substances used in metal extraction must be treated properly before being directed into rivers and streams. Alternatively, these materials may be drained into reservoirs where they can gradually percolate deep into the soil and evaporate into the air without causing much harm to the surrounding ecosystems. In some mines, absorbent carpets are spread on the surface of the ground to trap the toxic substances contained in liquid chemicals used for mining, hence preventing these chemicals from finding their way to water bodies.



































It is important for me thanks