Topic 7- Chemical Kinetics, Equilibrium and Energetics – Chemistry Form Three
The Rate of Chemical Reactions
- Addition of sodium metal to water: 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) The reaction takes place immediately and violently. It is therefore a fast reaction.
- The rusting of iron in the presence of air and water giving hydrated iron (III) oxide, F2O3.XH2O: This is an extremely slow reaction.
- the amount of zinc used up per unit of time;
- the amount of sulphuric acid used up per unit of time;
- the amount of zinc sulphate produced per unit of time; or
- the amount of hydrogen produced per unit of time.
Perform experiments to measure the rates of chemical reactions
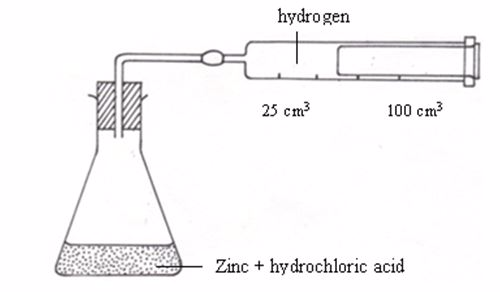
Apparatus for measuring the production of gas
| Time (s) | Volume of hydrogen gas (cm3) |
| 0 | 0 |
| 20 | 13 |
| 40 | 22 |
| 60 | 30 |
| 80 | 37 |
| 100 | 41 |
| 120 | 44 |
| 140 | 46 |
| 160 | 47 |
| 180 | 47 |
| 200 | 47 |
Questions from the experiment
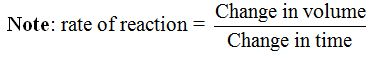
- change in intensity of colour: Many chemical reactions involve a change in colour. Potassium permanganate, for example, when it reacts with sulphur dioxide it changes from purple to colourless. The rate of such a reaction could be determined by measuring the rate at which the colour changes.
- formation or disappearance of a precipitate: The reaction between hydrochloric acid and sodium thiosulphate produce a yellow precipitate of sulphur. The rate at which this precipitate forms is a measure of the rate of a reaction.
Factors Affecting the Rate of Chemical Reactions
| Concentration of thiosulphate (g/dm3) | 10 | 20 | 30 | 40 | 50 |
| Time(s) for the cross to disappear | 250 | 120 | 65 | 32 | 15 |
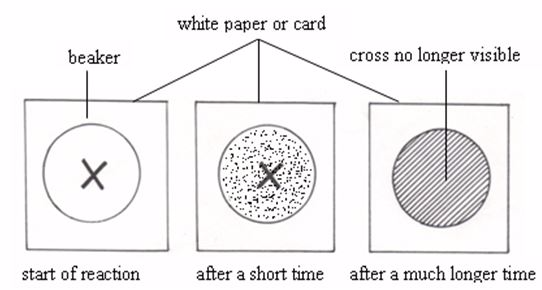
The Effect of Temperature on the Rate of a Reaction
Demonstrate the effect of temperature on the rate of a reaction
The reaction rate can be determined by the following procedure:
- 50 cm3 of a solution of sodium thiosulphate (containing 40g per litre of the solution) is measured and put into a 100 cm3 beaker.
- A wire gauze is placed on a tripod stand, then the beaker is put on the gauze, and the solution is gently warmed with a Bunsen burner flame.
- A thick black cross is marked on a white piece of paper or cardboard.
- When the temperature reaches a little above 200C, the beaker is taken quickly and placed on the cross.
- 10 cm3 of 2M hydrochloric acid solution is added quickly to the beaker and a clock is started at the same time. The temperature of the mixture is noted.
- When the cross is no longer visible, the clock is stopped.
- The experiment is repeated more times at temperatures of 300C, 400C, 500C, 600C, etc. Each time 10 cm3 of 2M hydrochloric acid and 50 cm3 of the thiosulphate solution are used.
Measuring the effect of temperature on the rate of reaction
The following table shows the specimen results obtained from such an experiment:
| Temperature (0C) | 20 | 30 | 40 | 50 | 60 |
| Time (s) for cross to disappear | 200 | 125 | 50 | 33 | 24 |
Show the effect of surface area of a solid on the rate of a reaction
One of the ways by which the rate of such a reaction can be increased is by reducing the particle size of the solid substance (marble chips). If this substance is grinded to fine powder or to small pellets, the surface area of the marble is increased. Therefore, more of the marble is exposed to the acid for efficient reaction. This leads to increase in the rate of reaction. Increased rate of reaction results to increased production of carbon dioxide gas.
Hence, the rate of reaction can be determined by measuring the time taken to produce a given volume of carbon dioxide by reacting equal masses of whole marble and crushed (or powdered) marble, and then comparing the two results. The data obtained is recorded in a table as shown below:
| Time (s) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
| Volume (cm3) | Whole marble | 0 | 18 | 24 | 32 | 38 | 45 | 53 | 62 | 72 | 80 | 80 |
| Grinded marble | 0 | 34 | 52 | 67 | 74 | 76 | 80 | 80 | 80 | 80 | 80 | |
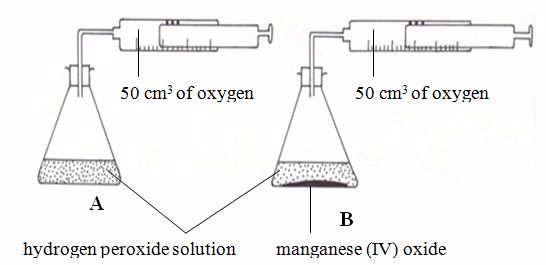
The Effect of Catalyst on the Rate of a Reaction
Demonstrate the effect of catalyst on the rate of a reaction
Investigating the effect of catalyst on rate of reaction
| Reaction | Catalyst |
| Heating of potassium chlorate 2KClO3(s) → 2KCl(s) + 3O2(g) | Manganese (IV) oxide (MnO2) |
| Synthesis of sulphur trioxide 2SO2(g) + O2(g)⇔2SO3(g) | Vanadium (IV) oxide (V2O5) |
|
Reduced iron powder |
|
Manganese (IV) oxide or platinum powder |
Reversible and Irreversible Reactions
- When you heat blue crystals of copper (II) sulphate, they breakdown into anhydrous copper (II) sulphate, a white powder: CuSO4.5H2O(s)⇌CuSO4(s) + 5H2O(g)The reaction can be reversed by just adding water to the white powder, which quickly turns to blue crystals again. In fact, this is used as a test for water: CuSO4(s) + 5H2O(l)⇌CuSO4.5H2O(s)
- When you heat ammonium chloride (a solid) in the bottom of a test tube, it breaks down into ammonia and hydrogen chloride (gases). The gases readily combine at the top of the tube where it is cool: NH4Cl(s)⇌NH3(g) + HCl(g)This is a reversible reaction.
Equilibrium Reactions
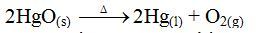
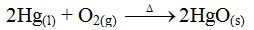
- Yield – how much yield is produced?
- Rate – how fast is it produced?
- Energy – how much energy is lost during the process?
THE HABER PROCESS FOR THE INDUSTRIAL MANUFACTURE OF AMMONIA
- N2 and H2 are mixed in the ration of 3:1
- An optimum temperature of 4500C is chosen.
- A very high pressure (200-500 atm) is applied.
- A catalyst of finely divided reduced iron, usually promoted by alumina (aluminium oxide) is used.
- The ammonia is condensed and removed out of the reaction mixture and the remaining N2and H2 recycled.
THE CONTACT PROCESS FOR THE INDUSTRIAL MANUFACTURE OF SULPHURIC ACID
The effect of pressure: There are fewer gas molecules on the right of the equation. Therefore, increasing the pressure would favour the production of sulphur trioxide. In fact, the process is run at atmospheric pressure because the conversion of sulphur dioxide to sulphur trioxide is about 96% complete under these conditions. In practice, it is found that use of high pressure produces a very small gain in yield and involves extra cost. It is for this reason that ordinary atmospheric pressure is used.
The effect of temperature: The reaction to produce sulphur trioxide is exothermic so, if the temperature is lowered the equilibrium shifts to the right. Hence, more sulphur trioxide will be produced. This means that sulphur trioxide production would be favoured by low temperatures. However, too low temperatures reduce the rate of reaction so increasing the time required for the production of the sulphur trioxide. This would mean an increase in production cost. For this reason, a catalyst must be introduced. A catalyst of vanadium (V) oxide is used to increase the rate of reaction. An optimum temperature of about 4500C is used. This gives sufficient sulphur trioxide at an economic rate. In general, low temperatures give an equilibrium favourable to an exothermic reaction, but catalysis is needed to give a favourable reaction rate.
Effect of concentration of reactants: Suppose that in the contact process reaction, an equilibrium has been reached in certain conditions and oxygen is added to the system. According to Le Chatelier’s principle, the reaction would shift to the right so as to oppose this change, that is, to reduce the concentration of the oxygen added towards its former level. This can only be achieved by combining it with sulphur dioxide to form sulphur trioxide. So, increased concentration of oxygen favours conversion of more sulphur dioxide to trioxide. Likewise, a similar case can occur when sulphur dioxide is added to the system. In a similar manner, increased concentration of sulphur trioxide would favour the conversion of oxygen to sulphur trioxide and hence increased formation of the trioxide.In general, the conditions used in the contact process are as follows: An optimum temperature of about 4500C is chosen; A catalyst, vanadium (V) oxide, is used to speed up the reaction; An operating pressure of 1 atmosphere is applied. A higher pressure, which would theoretically increase the yield of SO3, is not used as it is uneconomical; Yield of SO3 would be increased by increasing either SO2 or O2. The cheaper reactant is O2.
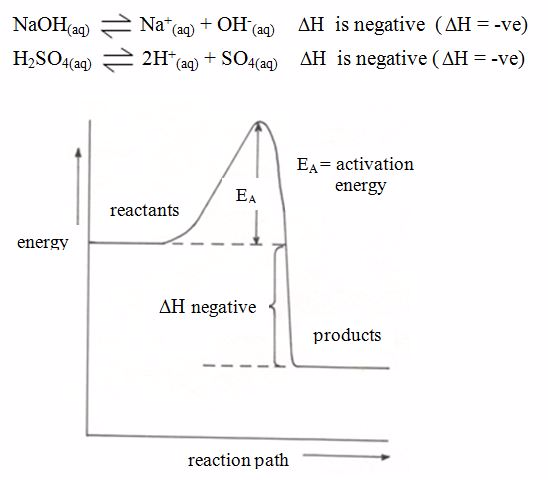
Endothermic and Exothermic Reaction
- An exothermic reaction is one during which heat is liberated to the surroundings.
- An endothermic reaction is one during which heat is absorbed from the surroundings
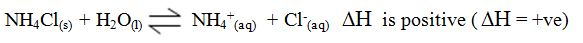
































Leave a Reply