Acids, Bases and Salts – Chemistry Form Three
Acids and Bases
| Source | Type of acid present |
| Mineral acids (HCl, H2SO4, HNO3, etc.) | Minerals |
| Tobacco | Salicylic acid |
| Tea | Tannic acid |
| Coffee | Chlorogenic acid |
| Sugar beet | Glutaric and adipic acids |
| Blackberry | Isocitric acid |
| Spinach, tomato | Oxalic acid |
| Sour (fermented milk) | Lactic acid |
| Bee, ant and nettle stings | Methanoic acid (formic acid) |
| Grapes, bananas, tamarinds | Tartaric acid |
| Citrus fruits | Citric acid ( lemons and limes have particularly high concentrations of citric acid; it can constitute as much as 8% of the dry weight of these fruits) |
| Indicator | Colour in acid | Colour in alkali (base) |
| Methyl orange | orange | yellow |
| Phenolphthalein | colourless | Pink |
| Litmus | red | blue |
| Bromothymol blue | yellow | blue |
Determine the reactions of acids with various materials
- Mg(s) + 2HNO3(aq) → Mg(NO3)2(aq) + H2(g)
- Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Characteristic reactions of bases
Most bases are insoluble in water. Some are soluble in water. Soluble bases are known as alkalis. The commonest alkalis are sodium hydroxide, (NaOH), calcium hydroxide, Ca(OH)2, potassium hydroxide, (KOH), and ammonium hydroxide or ammonia solution, (NH4OH). All alkaline solutions contain hydroxyl ions, OH–. In sodium hydroxide solution, the ions are produced like this:
- When sodium hydroxide solution is added to copper (II) sulphate solution, a pale blue precipitate of copper (II) hydroxide is formed. CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2SO4(aq)
- Another example is the reaction between potassium hydroxide and iron (II) chloride, which precipitates iron (II) hydroxide. FeCl3(aq) + 3KOH(aq) → Fe(OH)3(s) + 3KCl(aq)
Colours of the universal indicator in different acidic and alkaline solutions
| pH range | Colour | Strength |
| 1, 2, 3 | Red | Strongly acidic |
| 4 | Orange | |
| 5, 6 | Yellow | Weakly acidic |
| 7 | Green | Neutral |
| 8, 9 | Blue Indigo | Weakly alkaline |
| 10, 11, 12, 13, 14 | Purple/violet | Strongly alkaline |
Salts
The other source of sodium chloride is seawater. The salty taste of seawater is due to the presence of salts such as sodium chloride and magnesium bromide. However, there are many different types of salts present in seawater, though in small proportions, as shown in the table below (table 3.5)
| Salt | Formula | Percentage composition |
| Sodium chloride | NaCl | 2.72 |
| Magnesium chloride | MgCl2 | 0.38 |
| Magnesium sulphate | MgSO4 | 0.17 |
| Calcium sulphate | CaSO4 | 0.13 |
| Potassium chloride | KCl | 0.09 |
| Calcium chloride | CaCO3 | 0.01 |
| Magnesium bromide | MgBr2 | 0.01 |
Types of Salts
- they react with bases to form salts and water only. NaHSO4(aq) + NaOH(aq)→ Na2SO4(aq) + H2O(l)
- they react with carbonates to yield carbon dioxide.2NaHSO4(aq) + Na2CO3(aq)→ 2Na2SO4(aq) + H2O(l) + CO2(g)
- Basic copper carbonate, CuCO3.Cu(OH)2
- Basic lead carbonate (white lead), PbCO3.Pb(OH)2
- Basic magnesium chloride, MgCl2.Mg(OH)2
- Basic zinc chloride, ZnCl2.Zn(OH)2
The patterns of solubility for various types of salts
| Soluble salts | Insoluble salts |
| 1. All sodium, potassium and ammonium salts. | silver, mercury(I) and lead chlorides barium, lead (II) and calcium sulphates but other common carbonates are insoluble.but other common hydroxides are insoluble. |
| 2. All nitrates of metals | |
| 3. All chlorides except .………………….. | |
| 4. All sulphates except………………….. | |
| 5. Sodium, potassium, and ammonium carbonates…………………………… hydroxides………………………….…… | |
| 6. Sodium, potassium and ammonium |
Prepare salts in the laboratory
Preparation of soluble salts
- Reaction between an acid and an alkali: In this method, a dilute acid is added to an alkali in the appropriate volume ratio. The reaction between an acid and an alkali is termed as neutralization. For example, sodium chloride may be prepared by the following neutralization reaction:NaOH(aq)+ HCl(aq)→ NaCl(aq) + H2O(l). Both reactants are soluble, and no gas is given off during the reaction. So, it is difficult to know when the reaction is over. In this case, you have to use an indicator. A universal indicator or litmus could be used, but even better is phenolphthalein. This is pink in alkaline solution, but colourless in neutral or acidic solutions.
- Reaction of a metal with an acid: This is another general method for preparing salts. For example, zinc sulphate can be made by reacting dilute sulphuric acid with zinc:Zn(s) + H2SO4(aq)→ ZnSO4(aq) + H2(g). However, this method is not suitable for all metals or all acids. It is good for preparing salts of fairly reactive metals such as magnesium, aluminium, zinc and iron. However, the reactions of highly reactive metals like sodium, potassium and calcium with acids are very violent and dangerous. The reaction with lead is too slow. Copper, silver and gold do not react at all.
- Reaction of a metal oxide with an acid. Metal oxides, as you studied early, react with dilute acids to produce salts. Copper oxide is an insoluble base. Although copper will not react with dilute sulphuric acid, copper (II) oxide will. The salt that forms is copper (II) sulphate.CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)
- Reaction of a metal carbonate with an acid. The reaction between metal carbonates and dilute acids are accompanied with evolution of carbon dioxide gas. The evolution of a gas can be used to indicate when the reaction is over. An example of such reactions is the reaction between calcium carbonate and dilute hydrochloric acid. CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
General methods of preparing soluble salts
- Stage 1: An excess (more than enough) of the solid is added to the acid and allowed to react. Using an excess of the solid makes sure that all the acid used up. If it is not used up at this stage, the acid would become more concentrated when the water is evaporated later (stage 3).
- Stage 2: The excess solid is filtered out after the reaction is completed.
- Stage 3: The filtrate is gently evaporated to concentrate the solution. This can be done on a heated water bath. Do not heat so strongly or “spitting” might take place.
- Stage 4: The concentrated solution is cooled down to let the crystals form. Filter off the crystals. Wash them with a little distilled water. Dry the crystals carefully between the filter papers.
General methods for preparing insoluble salts
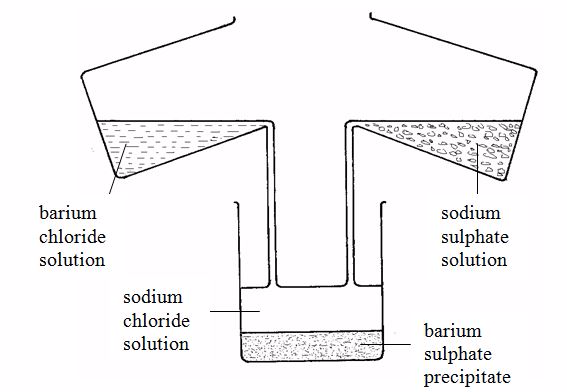
Preparation of salts by direct combination (synthesis)
Examine the effects of heat on salts
When different salts are heated, they behave in different manners. The crystals of some salts contain water of crystallization. When these hydrated salts are heated, their water of crystallization is driven off as steam. The crystals then lose their shape and become a powder. The following are few examples of hydrated salts:
| Salt formula | Chemical name |
| CuSO4.5H2O | Copper (II) sulphate five water |
| Na2CO3.I0H2O | Sodium carbonate ten water |
| MgCl2.6H2O | Magnesium chloride six water |
| FeCl3.6H2O | Iron (III) chloride six water |
| FeSO4.7H2O | Iron (II) sulphate seven water |
| CoCl2.6H2O | Cobalt (II) chloride six water |
| MgSO4.7H2O | Magnesium sulphate seven water |
| CaSO4.2H2O | Calcium sulphate two water |
Sulphates
Chlorides
Carbonates and hydrogencarbonates
DELIQUESCENCE, EFFLORESCENCE AND HYGROSCOPY
Calcium carbonate (marble, limestone, chalk)
- An important use of calcium carbonate is in the building industry. It is widely used in making cement, lime, mortar and making steel from iron.
- Powdered limestone is used as a liming material to neutralize soil acidity. When used in this way, it is termed as agricultural lime. When added in the soil, agricultural lime acts as a calcium source for plants as well as increasing the pH and water retaining capacity of acidic soils.
- It is also used in making paint, plastic, rubber, ceramic and glass; and in oil refining, and iron ore purification.
- Calcium carbonate is the most preferred mineral in the paper industry. It helps in the production of the best quality papers.
- Since calcium is essential for healthy bones and teeth, it is used as a dietary calcium supplement.
- Limestone can also be well shaped, painted, and then used as decorative stones.

































Leave a Reply